J Gastric Cancer.
2017 Mar;17(1):88-92. 10.5230/jgc.2017.17.e9.
Natural History of Early Gastric Cancer: a Case Report and Literature Review
- Affiliations
-
- 1Division of Endoscopy, Shizuoka Cancer Center, Shizuoka, Japan. kakushin-tky@umin.ac.jp
- KMID: 2372585
- DOI: http://doi.org/10.5230/jgc.2017.17.e9
Abstract
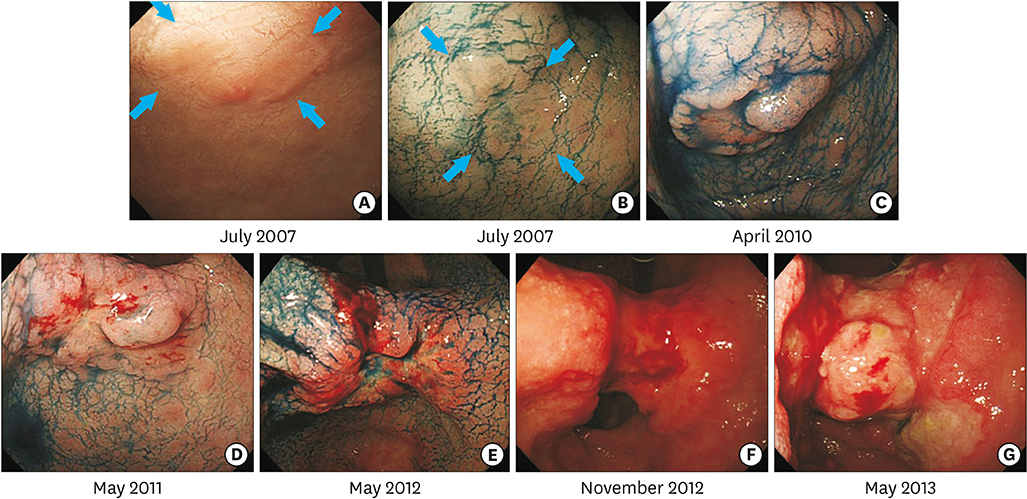
- Early detection and treatment decrease the mortality rate associated with gastric cancer (GC). However, the natural history of GC remains unclear. An 85-year-old woman was referred to our hospital for evaluation of a gastric tumor. Esophagogastroduodenoscopy identified a 6 mm, flat-elevated lesion at the lesser curvature of the antrum. A biopsy specimen showed a well-differentiated tubular adenocarcinoma. The depth of the lesion was estimated to be intramucosal. Although the lesion met the indications for endoscopic resection, periodic endoscopic follow-up was performed due to the patient's advanced age and comorbidities. The mucosal GC invaded into the submucosa 3 years later, and finally progressed to advanced cancer 5 years after the initial examination. The patient died of tumor hemorrhage 6.4 years after the initial examination. In this case, mucosal GC progressed to advanced GC, eventually leading to the patient's death from GC. Early and appropriate treatment is required to prevent GC-related death.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Trends and Outcomes of Non-compliance with Treatment for Gastric Cancer in Korea over the 16 years from 1999 to 2015
Bang Wool Eom, Kyu-Won Jung, Young-Joo Won, Young-Woo Kim
J Gastric Cancer. 2019;19(1):92-101. doi: 10.5230/jgc.2019.19.e5.
Reference
-
1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90.2. Marchet A, Mocellin S, Ambrosi A, Morgagni P, Vittimberga G, Roviello F, et al. Validation of the new AJCC TNM staging system for gastric cancer in a large cohort of patients (n = 2,155): focus on the T category. Eur J Surg Oncol. 2011; 37:779–785.3. Nashimoto A, Akazawa K, Isobe Y, Miyashiro I, Katai H, Kodera Y, et al. Gastric cancer treated in 2002 in Japan: 2009 annual report of the JGCA nationwide registry. Gastric Cancer. 2013; 16:1–27.4. Sekiguchi M, Suzuki H, Oda I, Abe S, Nonaka S, Yoshinaga S, et al. Favorable long-term outcomes of endoscopic submucosal dissection for locally recurrent early gastric cancer after endoscopic resection. Endoscopy. 2013; 45:708–713.5. Isomoto H, Shikuwa S, Yamaguchi N, Fukuda E, Ikeda K, Nishiyama H, et al. Endoscopic submucosal dissection for early gastric cancer: a large-scale feasibility study. Gut. 2009; 58:331–336.6. Tsukuma H, Mishima T, Oshima A. Prospective study of “early” gastric cancer. Int J Cancer. 1983; 31:421–426.7. Tsukuma H, Oshima A, Narahara H, Morii T. Natural history of early gastric cancer: a non-concurrent, long term, follow up study. Gut. 2000; 47:618–621.8. Lansdown M, Quirke P, Dixon MF, Axon AT, Johnston D. High grade dysplasia of the gastric mucosa: a marker for gastric carcinoma. Gut. 1990; 31:977–983.9. Everett SM, Axon AT. Early gastric cancer: disease or pseudo-disease? Lancet. 1998; 351:1350–1352.10. Rugge M, Cassaro M, Di Mario F, Leo G, Leandro G, Russo VM, Interdisciplinary Group on Gastric Epithelial Dysplasia (IGGED), et al. The long term outcome of gastric non-invasive neoplasia. Gut. 2003; 52:1111–1116.11. Kaneko E, Nakamura T, Umeda N, Fujino M, Niwa H. Outcome of gastric carcinoma detected by gastric mass survey in Japan. Gut. 1977; 18:626–630.12. Tashiro A, Sano M, Kinameri K, Fujita K, Takeuchi Y. Comparing mass screening techniques for gastric cancer in Japan. World J Gastroenterol. 2006; 12:4873–4874.13. Yoshida M, Shimoda T, Kusafuka K, Sugino T, Nakajima T, Ono H. Comparative study of Western and Japanese criteria for biopsy-based diagnosis of gastric epithelial neoplasia. Gastric Cancer. 2015; 18:239–245.14. Fujisaki J, Nakajima T, Hirasawa T, Yamamoto Y, Ishiyama A, Tsuchida T, et al. Natural history of gastric cancer-a case followed up for eight years: early to advanced gastric cancer. Clin J Gastroenterol. 2012; 5:351–354.15. Furukawa K, Yamada K, Konomi K, Tanaka M. Gastric carcinoma resected 95 months after being diagnosed: report of a case. Surg Today. 1994; 24:756–758.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Natural History of Untreated Early Gastric Cancer
- A Case of Synchronous Double Early Gastric Cancer Lasting for 10 Years in Elderly Patient
- A Case of Early Gastric Cancer in Childhood
- Early Cancer of the Gastric Stump after Gastrojejunostomy for Duodenal Ulcer Obstruction
- Esophagus, Stomach & Intestine; One Case of Early Gastric Stump Cancer Following Partial Gastrectomy for Gastroptosis