J Gastric Cancer.
2015 Jun;15(2):113-120. 10.5230/jgc.2015.15.2.113.
Effects of Continuing Adjuvant S-1 for 1 Year on the Prognosis of Gastric Cancer Patients: Results from a Prospective Single Center Study
- Affiliations
-
- 1Division of Gastrointestinal Surgery, Department of Surgery, Ajou University School of Medicine, Suwon, Korea. hhcmc75@naver.com
- KMID: 2372358
- DOI: http://doi.org/10.5230/jgc.2015.15.2.113
Abstract
- PURPOSE
Although several clinical trials have proven the efficacy of adjuvant S-1 treatment in gastric cancers, it is still unclear which patients receive the most benefit. In this study, we prospectively recruited patients with locally advanced gastric cancer who had undergone curative resection followed by adjuvant S-1 administration to investigate which factors affect the outcomes.
MATERIALS AND METHODS
Between July 2010 and October 2011, we enrolled 49 patients who underwent curative resection for stage II or III gastric cancer and who subsequently received adjuvant S-1 treatment for 1 year.
RESULTS
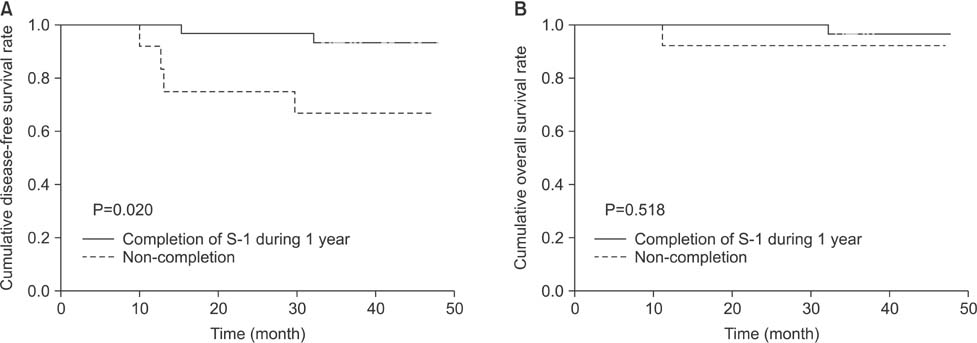
Twenty-nine patients (59.2%) continued S-1 treatment for 1 year, and 12 patients (24.5%) experienced recurrent disease during the follow-up period. Patients with continuation of S-1 for 1 year had significantly increased rates of disease-free survival (P<0.001) and overall survival (P=0.001) relative to the patients who discontinued S-1 during year 1. Multivariate analysis indicated poor outcomes for patients with stage III disease and those who discontinued S-1 treatment. Excluding patients who discontinued S-1 due to cancer progression (n=7), adjuvant treatment with S-1 still demonstrated a significant difference in the disease-free survival rate between the patients who continued treatment and those who discontinued it (P=0.020).
CONCLUSIONS
S-1 is tolerated as adjuvant treatment in gastric cancer patients. However, discontinuing S-1 treatment may be an unfavorable factor in the prevention of recurrence. S-1 adjuvant treatment should be continued for 1 year if possible through the proper management of toxicities.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
The Prognostic Significance of Compliance with Postoperative Adjuvant Chemotherapy in Patients with Stage III Gastric Cancer: an Observational Study
Sung Ho Jang, Young Jae Jung, Min Gyu Kim, Sung Joon Kwon
J Gastric Cancer. 2018;18(1):48-57. doi: 10.5230/jgc.2018.18.e4.
Reference
-
1. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015; 136:E359–E386.2. Hochwald SN, Brennan MF, Klimstra DS, Kim S, Karpeh MS. Is lymphadenectomy necessary for early gastric cancer? Ann Surg Oncol. 1999; 6:664–670.3. Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006; 355:11–20.4. Earle CC, Maroun JA. Adjuvant chemotherapy after curative resection for gastric cancer in non-Asian patients: revisiting a meta-analysis of randomised trials. Eur J Cancer. 1999; 35:1059–1064.5. Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001; 345:725–730.6. Markar SR, Wiggins T, Ni M, Steyerberg EW, Van Lanschot JJ, Sasako M, et al. Assessment of the quality of surgery within randomised controlled trials for the treatment of gastrooesophageal cancer: a systematic review. Lancet Oncol. 2015; 16:e23–e31.7. Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012; 379:315–321.8. Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007; 357:1810–1820.9. Shirasaka T, Shimamato Y, Ohshimo H, Yamaguchi M, Kato T, Yonekura K, et al. Development of a novel form of an oral 5-fluorouracil derivative (S-1) directed to the potentiation of the tumor selective cytotoxicity of 5-fluorouracil by two biochemical modulators. Anticancer Drugs. 1996; 7:548–557.10. Aoyama T, Yoshikawa T, Shirai J, Hayashi T, Yamada T, Tsuchida K, et al. Body weight loss after surgery is an independent risk factor for continuation of S-1 adjuvant chemotherapy for gastric cancer. Ann Surg Oncol. 2013; 20:2000–2006.11. Iwasa S, Yamada Y, Fukagawa T, Eguchi Nakajima T, Kato K, Hamaguchi T, et al. Management of adjuvant S-1 therapy after curative resection of gastric cancer: dose reduction and treatment schedule modification. Gastric Cancer. 2011; 14:28–34.12. Kim SJ, Kim K, Park Y, Kim BS, Huh J, Ko YH, et al. Dose modification of alemtuzumab in combination with dexamethasone, cytarabine, and cisplatin in patients with relapsed or refractory peripheral T-cell lymphoma: analysis of efficacy and toxicity. Invest New Drugs. 2012; 30:368–375.13. Aoyama T, Yoshikawa T, Hayashi T, Kuwabara H, Mikayama Y, Ogata T, et al. Risk factors for 6-month continuation of S-1 adjuvant chemotherapy for gastric cancer. Gastric Cancer. 2013; 16:133–139.14. Jeong JH, Ryu MH, Ryoo BY, Lee SS, Park I, Lee SH, et al. Safety and feasibility of adjuvant chemotherapy with S-1 for Korean patients with curatively resected advanced gastric cancer. Cancer Chemother Pharmacol. 2012; 70:523–529.15. Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003; 13:176–181.16. Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011; 29:4387–4393.17. Kochi M, Fujii M, Kanamori N, Kaiga T, Aizaki K, Takahashi T, et al. Effect of gastrectomy on the pharmacokinetics of S-1, an oral fluoropyrimidine, in resectable gastric cancer patients. Cancer Chemother Pharmacol. 2007; 60:693–701.18. Benson AB 3rd, Ajani JA, Catalano RB, Engelking C, Kornblau SM, Martenson JA Jr, et al. Recommended guidelines for the treatment of cancer treatment-induced diarrhea. J Clin Oncol. 2004; 22:2918–2926.19. Gibson RJ, Stringer AM. Chemotherapy-induced diarrhoea. Curr Opin Support Palliat Care. 2009; 3:31–35.20. Keefe DM, Gibson RJ, Hauer-Jensen M. Gastrointestinal mucositis. Semin Oncol Nurs. 2004; 20:38–47.21. Keefe DM. Intestinal mucositis: mechanisms and management. Curr Opin Oncol. 2007; 19:323–327.22. Keefe DM, Brealey J, Goland GJ, Cummins AG. Chemotherapy for cancer causes apoptosis that precedes hypoplasia in crypts of the small intestine in humans. Gut. 2000; 47:632–637.23. McCollum AD, Catalano PJ, Haller DG, Mayer RJ, Macdonald JS, Benson AB 3rd, et al. Outcomes and toxicity in africanamerican and caucasian patients in a randomized adjuvant chemotherapy trial for colon cancer. J Natl Cancer Inst. 2002; 94:1160–1167.24. Zalcberg J, Kerr D, Seymour L, Palmer M. Haematological and non-haematological toxicity after 5-fluorouracil and leucovorin in patients with advanced colorectal cancer is significantly associated with gender, increasing age and cycle number. Tomudex International Study Group. Eur J Cancer. 1998; 34:1871–1875.25. Mattison LK, Fourie J, Hirao Y, Koga T, Desmond RA, King JR, et al. The uracil breath test in the assessment of dihydropyrimidine dehydrogenase activity: pharmacokinetic relationship between expired 13CO2 and plasma [2-13C]dihydrouracil. Clin Cancer Res. 2006; 12:549–555.26. Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008; 9:215–221.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Role of Adjuvant Radiotherapy in Gastric Cancer
- Update of Adjuvant Chemotherapy for Resected Gastric Cancer
- Screening of gastric cancer
- Current Status of Chemotherapy for Gastric Cancer Patients in Korea - A Nationwide Survey -
- Postoperative Adjuvant Radiotherapy for Patients with Gastric Adenocarcinoma