Allergy Asthma Immunol Res.
2017 May;9(3):237-246. 10.4168/aair.2017.9.3.237.
Anti-Interleukin-9 Antibody Increases the Effect of Allergen-Specific Immunotherapy in Murine Allergic Rhinitis
- Affiliations
-
- 1Department of Otolaryngology-Head and Neck Surgery, College of Medicine, The Catholic University of Korea, Seoul, Korea. kshent@catholic.ac.kr
- KMID: 2371724
- DOI: http://doi.org/10.4168/aair.2017.9.3.237
Abstract
- PURPOSE
Interleukin (IL)-9 induces allergic responses; however, the roles of anti-IL-9 antibody in the induction of tolerance remain unclear. This study investigated the effects of anti-IL-9 antibody on oral tolerance (OT) in a mouse model of allergic rhinitis (AR).
METHODS
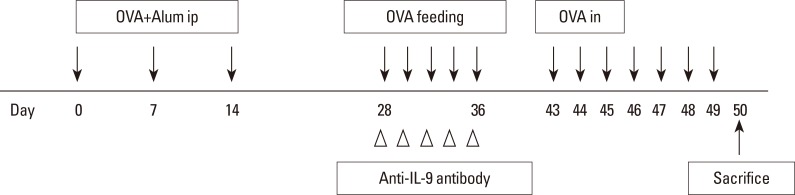
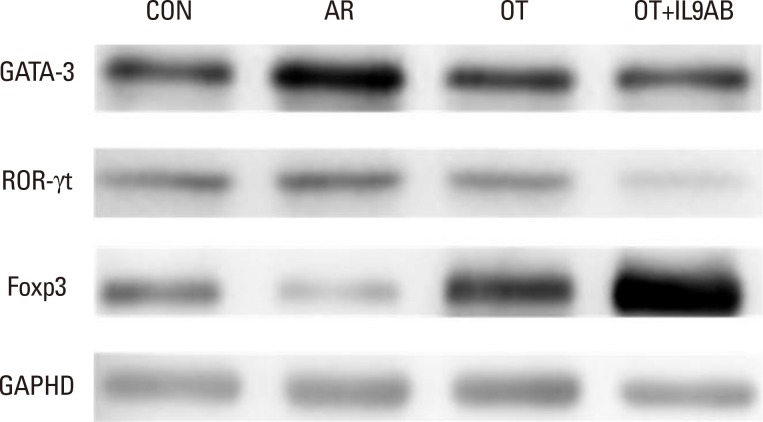
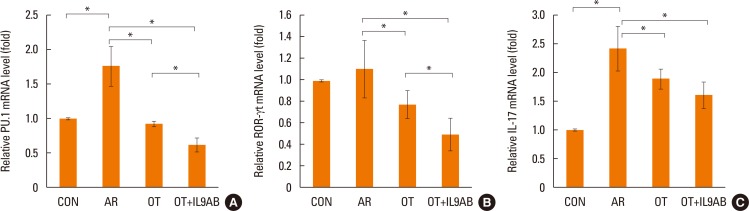
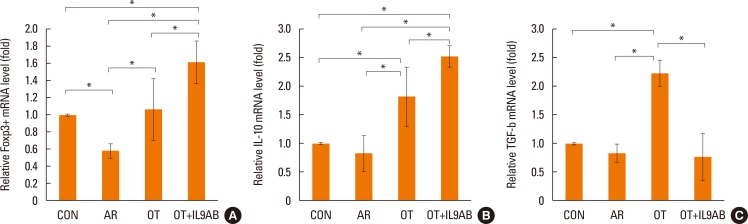
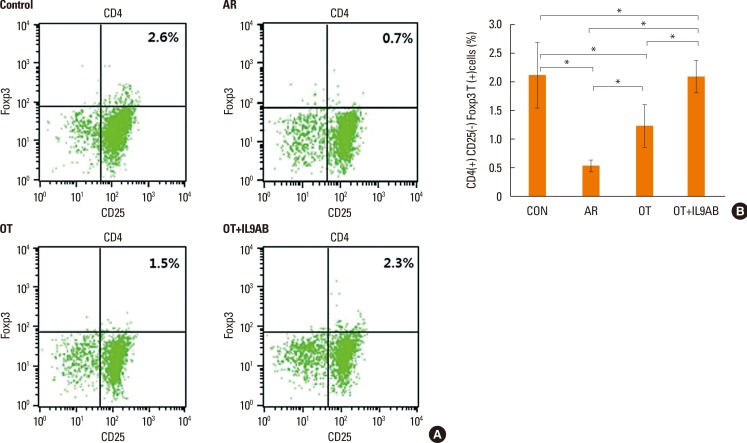
BALB/c mice were divided into 4 groups: the control, AR, OT, and OT with anti-IL-9 antibody (OT+IL9AB) groups. Ovalbumin (OVA) was used for sensitization and challenge. Mice in the OT and OT+IL9AB groups were fed OVA for immunotherapy. During immunotherapy, OT+IL9AB mice were injected with anti-IL-9 antibody. Allergic symptoms, tissue eosinophil counts, and serum OVA-specific immunoglobulin E (IgE) were measured. The mRNA expressions of cytokines and transcription factors of T cells of nasal mucosa were determined by real-time polymerase chain reaction (PCR). The protein levels of GATA3, ROR-γt, and Foxp3 in nasal mucosa were determined by Western blot. CD4âºCD25âºFoxp3⺠T cells in the spleen were analyzed by flow cytometry.
RESULTS
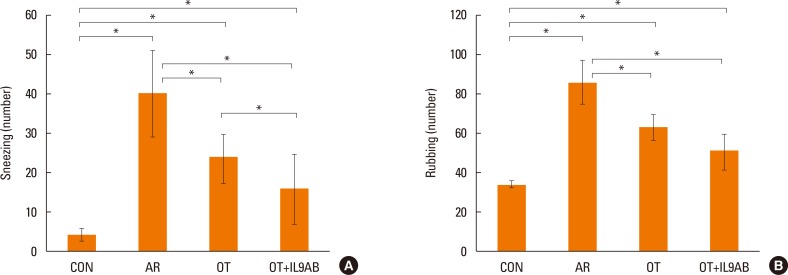
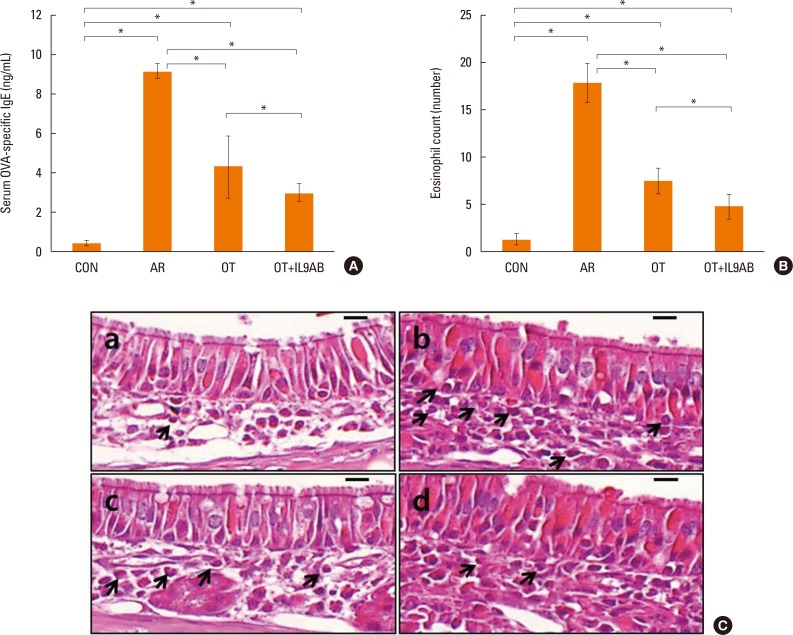
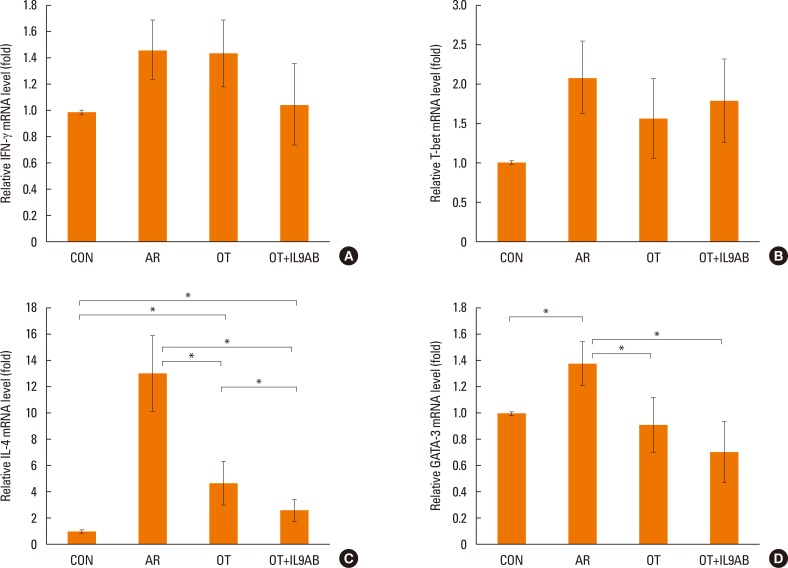
Administration of anti-IL-9 antibody decreased allergic symptoms, OVA-specific IgE levels, and eosinophil counts. In addition, it inhibited T-helper (Th) 2 responses, but had no effect on Th1 responses. Protein levels of ROR-γt and mRNA levels of PU.1 and ROR-γt were reduced by anti-IL-9 antibody. Anti-IL-9 antibody increased Foxp3 and IL-10 mRNA expression, Foxp3 protein, and induction of CD4âºCD25âºFoxp3⺠T cells.
CONCLUSIONS
Anti-IL-9 antibody decreased allergic inflammation through suppression of Th2 and Th17 cells. Anti-IL-9 antibody enhanced the tolerogenic effects of regulatory T cells. These results suggest that anti-IL-9 antibody might represent a potential therapeutic agent for allergen immunotherapy in patients with uncontrolled allergic airway disease.
MeSH Terms
-
Animals
Blotting, Western
Cytokines
Desensitization, Immunologic
Eosinophils
Flow Cytometry
Humans
Immunoglobulin E
Immunoglobulins
Immunotherapy*
Inflammation
Interleukin-10
Interleukin-9
Interleukins
Mice
Nasal Mucosa
Ovalbumin
Ovum
Real-Time Polymerase Chain Reaction
Rhinitis, Allergic*
RNA, Messenger
Spleen
T-Lymphocytes
T-Lymphocytes, Regulatory
Th17 Cells
Transcription Factors
Cytokines
Immunoglobulin E
Immunoglobulins
Interleukin-10
Interleukin-9
Interleukins
Ovalbumin
RNA, Messenger
Transcription Factors
Figure
Reference
-
1. Price D, Smith P, Hellings P, Papadopoulos N, Fokkens W, Muraro A, et al. Current controversies and challenges in allergic rhinitis management. Expert Rev Clin Immunol. 2015; 11:1205–1217. PMID: 26325631.
Article2. Ciprandi G, Marseglia GL, Castagnoli R, Valsecchi C, Tagliacarne C, Caimmi S, et al. From IgE to clinical trials of allergic rhinitis. Expert Rev Clin Immunol. 2015; 11:1321–1333. PMID: 26358006.
Article3. Kim CH, Kim JK, Kim HJ, Cho JH, Kim JS, Kim YD, et al. Comparison of intranasal ciclesonide, oral levocetirizine, and combination treatment for allergic rhinitis. Allergy Asthma Immunol Res. 2015; 7:158–166. PMID: 25729623.
Article4. Burks AW, Calderon MA, Casale T, Cox L, Demoly P, Jutel M, et al. Update on allergy immunotherapy: American Academy of Allergy, Asthma & Immunology/European Academy of Allergy and Clinical Immunology/PRACTALL consensus report. J Allergy Clin Immunol. 2013; 131:1288–1296.e3. PMID: 23498595.5. Jutel M, Kosowska A, Smolinska S. Allergen immunotherapy: past, present, and future. Allergy Asthma Immunol Res. 2016; 8:191–197. PMID: 26922928.
Article6. Akdis CA. Therapies for allergic inflammation: refining strategies to induce tolerance. Nat Med. 2012; 18:736–749. PMID: 22561837.
Article7. Kim YM, Kim YS, Jeon SG, Kim YK. Immunopathogenesis of allergic asthma: more than the Th2 hypothesis. Allergy Asthma Immunol Res. 2013; 5:189–196. PMID: 23814671.
Article8. Pawankar R, Hayashi M, Yamanishi S, Igarashi T. The paradigm of cytokine networks in allergic airway inflammation. Curr Opin Allergy Clin Immunol. 2015; 15:41–48. PMID: 25479317.
Article9. Wisniewski JA, Borish L. Novel cytokines and cytokine-producing T cells in allergic disorders. Allergy Asthma Proc. 2011; 32:83–94. PMID: 21439160.
Article10. Kaplan MH, Hufford MM, Olson MR. The development and in vivo function of T helper 9 cells. Nat Rev Immunol. 2015; 15:295–307. PMID: 25848755.
Article11. Wilhelm C, Turner JE, Van Snick J, Stockinger B. The many lives of IL-9: a question of survival? Nat Immunol. 2012; 13:637–641. PMID: 22713829.
Article12. Schmitt E, Klein M, Bopp T. Th9 cells, new players in adaptive immunity. Trends Immunol. 2014; 35:61–68. PMID: 24215739.
Article13. Wynn TA. Type 2 cytokines: mechanisms and therapeutic strategies. Nat Rev Immunol. 2015; 15:271–282. PMID: 25882242.
Article14. Sehra S, Yao W, Nguyen ET, Glosson-Byers NL, Akhtar N, Zhou B, et al. TH9 cells are required for tissue mast cell accumulation during allergic inflammation. J Allergy Clin Immunol. 2015; 136:433–440.e1. PMID: 25746972.
Article15. Boyman O, Kaegi C, Akdis M, Bavbek S, Bossios A, Chatzipetrou A, et al. EAACI IG biologicals task force paper on the use of biologic agents in allergic disorders. Allergy. 2015; 70:727–754. PMID: 25819018.
Article16. Staudt V, Bothur E, Klein M, Lingnau K, Reuter S, Grebe N, et al. Interferon-regulatory factor 4 is essential for the developmental program of T helper 9 cells. Immunity. 2010; 33:192–202. PMID: 20674401.
Article17. Oh CK, Leigh R, McLaurin KK, Kim K, Hultquist M, Molfino NA. A randomized, controlled trial to evaluate the effect of an anti-interleukin-9 monoclonal antibody in adults with uncontrolled asthma. Respir Res. 2013; 14:93. PMID: 24050312.
Article18. Parker JM, Oh CK, LaForce C, Miller SD, Pearlman DS, Le C, et al. Safety profile and clinical activity of multiple subcutaneous doses of MEDI-528, a humanized anti-interleukin-9 monoclonal antibody, in two randomized phase 2a studies in subjects with asthma. BMC Pulm Med. 2011; 11:14. PMID: 21356110.
Article20. Sato MN, Carvalho AF, Silva AO, MacIel M Jr, Fusaro AE, Duarte AJ. Oral tolerance induced to house dust mite extract in naive and sensitized mice: evaluation of immunoglobulin G anti-immunoglobulin E autoantibodies and IgG-IgE complexes. Immunology. 1998; 95:193–199. PMID: 9824475.
Article21. Strobel S. Oral tolerance, systemic immunoregulation, and autoimmunity. Ann N Y Acad Sci. 2002; 958:47–58. PMID: 12021083.
Article22. Ruiz Schütz VC, Drewiacki T, Nakashima AS, Arantes-Costa FM, Prado CM, Kasahara DI, et al. Oral tolerance attenuates airway inflammation and remodeling in a model of chronic pulmonary allergic inflammation. Respir Physiol Neurobiol. 2009; 165:13–21. PMID: 18930843.
Article23. Kerzerho J, Maazi H, Speak AO, Szely N, Lombardi V, Khoo B, et al. Programmed cell death ligand 2 regulates TH9 differentiation and induction of chronic airway hyperreactivity. J Allergy Clin Immunol. 2013; 131:1048–1057. 1057.e1–1057.e2. PMID: 23174661.
Article24. Akdis CA, Akdis M. Mechanisms of allergen-specific immunotherapy and immune tolerance to allergens. World Allergy Organ J. 2015; 8:17. PMID: 26023323.
Article25. Nowak EC, Noelle RJ. Interleukin-9 and T cell subsets. Cell Cycle. 2009; 8:3798–3799. PMID: 19934658.
Article26. Chung Y, Cho J, Chang YS, Cho SH, Kang CY. Preventive and therapeutic effects of oral tolerance in a murine model of asthma. Immunobiology. 2002; 206:408–423. PMID: 12437071.
Article27. Keller AC, Mucida D, Gomes E, Faquim-Mauro E, Faria AM, Rodriguez D, et al. Hierarchical suppression of asthma-like responses by mucosal tolerance. J Allergy Clin Immunol. 2006; 117:283–290. PMID: 16461128.
Article28. Shin JH, Kang JM, Kim SW, Cho JH, Park YJ, Kim SW. Effect of oral tolerance in a mouse model of allergic rhinitis. Otolaryngol Head Neck Surg. 2010; 142:370–375. PMID: 20172383.
Article29. Adel-Patient K, Wavrin S, Bernard H, Meziti N, Ah-Leung S, Wal JM. Oral tolerance and Treg cells are induced in BALB/c mice after gavage with bovine β-lactoglobulin. Allergy. 2011; 66:1312–1321. PMID: 21615416.
Article30. Farahani R, Sherkat R, Hakemi MG, Eskandari N, Yazdani R. Cytokines (interleukin-9, IL-17, IL-22, IL-25 and IL-33) and asthma. Adv Biomed Res. 2014; 3:127. PMID: 24949298.
Article31. van den Brûle S, Heymans J, Havaux X, Renauld JC, Lison D, Huaux F, et al. Profibrotic effect of IL-9 overexpression in a model of airway remodeling. Am J Respir Cell Mol Biol. 2007; 37:202–209. PMID: 17446528.
Article32. Kearley J, Erjefalt JS, Andersson C, Benjamin E, Jones CP, Robichaud A, et al. IL-9 governs allergen-induced mast cell numbers in the lung and chronic remodeling of the airways. Am J Respir Crit Care Med. 2011; 183:865–875. PMID: 20971830.
Article33. Chang HC, Sehra S, Goswami R, Yao W, Yu Q, Stritesky GL, et al. The transcription factor PU.1 is required for the development of IL-9-producing T cells and allergic inflammation. Nat Immunol. 2010; 11:527–534. PMID: 20431622.
Article34. Kim MS, Cho KA, Cho YJ, Woo SY. Effects of interleukin-9 blockade on chronic airway inflammation in murine asthma models. Allergy Asthma Immunol Res. 2013; 5:197–206. PMID: 23814672.
Article35. Jones CP, Gregory LG, Causton B, Campbell GA, Lloyd CM. Activin A and TGF-beta promote T(H)9 cell-mediated pulmonary allergic pathology. J Allergy Clin Immunol. 2012; 129:1000–1010.e3. PMID: 22277204.36. Hoppenot D, Malakauskas K, Lavinskienė S, Bajoriūnienė I, Kalinauskaitė V, Sakalauskas R. Peripheral blood Th9 cells and eosinophil apoptosis in asthma patients. Medicina (Kaunas). 2015; 51:10–17. PMID: 25744770.
Article37. Sherkat R, Yazdani R, Ganjalikhani Hakemi M, Homayouni V, Farahani R, Hosseini M, et al. Innate lymphoid cells and cytokines of the novel subtypes of helper T cells in asthma. Asia Pac Allergy. 2014; 4:212–221. PMID: 25379481.
Article38. Yao W, Tepper RS, Kaplan MH. Predisposition to the development of IL-9-secreting T cells in atopic infants. J Allergy Clin Immunol. 2011; 128:1357–1360.e5. PMID: 21798577.
Article39. Froidure A, Shen C, Gras D, Van Snick J, Chanez P, Pilette C. Myeloid dendritic cells are primed in allergic asthma for thymic stromal lymphopoietin-mediated induction of Th2 and Th9 responses. Allergy. 2014; 69:1068–1076. PMID: 24888572.
Article40. Yao W, Zhang Y, Jabeen R, Nguyen ET, Wilkes DS, Tepper RS, et al. Interleukin-9 is required for allergic airway inflammation mediated by the cytokine TSLP. Immunity. 2013; 38:360–372. PMID: 23376058.
Article41. Licona-Limón P, Kim LK, Palm NW, Flavell RA. TH2, allergy and group 2 innate lymphoid cells. Nat Immunol. 2013; 14:536–542. PMID: 23685824.
Article42. Hoppenot D, Malakauskas K, Lavinskiene S, Sakalauskas R. p-STAT6, PU.1, and NF-kappaB are involved in allergen-induced late-phase airway inflammation in asthma patients. BMC Pulm Med. 2015; 15:122. PMID: 26466682.
Article43. Elyaman W, Bradshaw EM, Uyttenhove C, Dardalhon V, Awasthi A, Imitola J, et al. IL-9 induces differentiation of TH17 cells and enhances function of FoxP3+ natural regulatory T cells. Proc Natl Acad Sci U S A. 2009; 106:12885–12890. PMID: 19433802.
Article44. Li H, Nourbakhsh B, Ciric B, Zhang GX, Rostami A. Neutralization of IL-9 ameliorates experimental autoimmune encephalomyelitis by decreasing the effector T cell population. J Immunol. 2010; 185:4095–4100. PMID: 20805418.
Article45. Lu LF, Lind EF, Gondek DC, Bennett KA, Gleeson MW, Pino-Lagos K, et al. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature. 2006; 442:997–1002. PMID: 16921386.
Article46. Li H, Nourbakhsh B, Cullimore M, Zhang GX, Rostami A. IL-9 is important for T-cell activation and differentiation in autoimmune inflammation of the central nervous system. Eur J Immunol. 2011; 41:2197–2206. PMID: 21674475.
Article