Ann Hepatobiliary Pancreat Surg.
2017 Feb;21(1):1-10. 10.14701/ahbps.2017.21.1.1.
Oral glucose tolerance test for preoperative assessment of liver function in liver resection
- Affiliations
-
- 1Department of Gastrointestinal Surgery, Govind Ballabh Pant Institute of Postgraduate Medical Education and Research, New Delhi, India. mnsaravanan@rediffmail.com
- 2National Institute of Gastroenterology and Liver Diseases, Hyderabad, India.
- KMID: 2371561
- DOI: http://doi.org/10.14701/ahbps.2017.21.1.1
Abstract
- BACKGROUNDS/AIMS
We intended to determine the role of the Oral glucose tolerance test (OGTT), in addition to volumetry, in preoperative assessment of patients undergoing liver resection.
METHODS
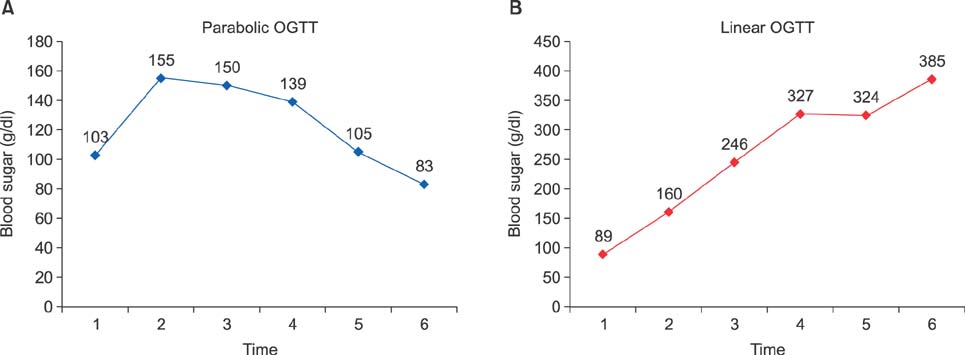
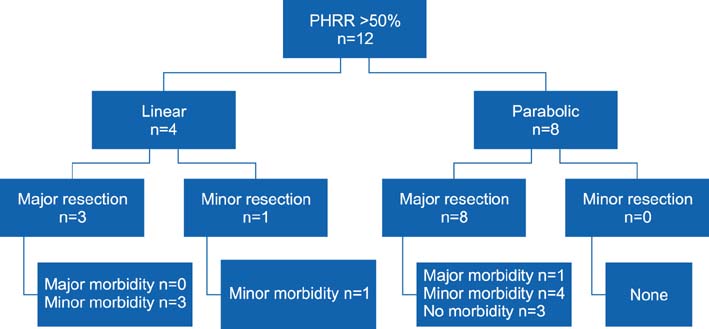
This was a prospective study conducted at a tertiary care hospital, between February 2009 and February 2011. OGTT curve (parabolic/linear), linearity index (LI) and Parenchymal Hepatic Resection Rate (PHRR) were correlated with postoperative outcomes in terms of postoperative liver failure (PLF), by 50-50 criteria, morbidity, mortality and hospital stay.
RESULTS
Of the 33 patients included in the study, 23 (69.7%) patients underwent major liver resections. Hepatocellular carcinoma (30.3%) was the leading indication. The overall postoperative morbidity rate was 72.7%, but major complications occurred in 3 (9.1%) patients only. There was no 90-day mortality. The 50-50 criteria were met by 3 patients undergoing major resection. Significant correlation was noted between the linear OGTT curve and the overall hospital stay (12.1 days vs. 9.6 days in parabolic; p=0.04). Patients with linear OGTT met the 50-50 criteria more often (18%) than those having a parabolic curve (4.5%; p=0.25). Although the OGTT was more often linear with occurrence of morbidity (41.7% vs 11.1%), major morbidity (66.7% vs 30%) and PLF by 50-50 criteria (66.7% vs 30%), it was not statistically significant. The linearity index was marginally lower (0.9 vs 1.2) in the presence of major morbidity and PLF by 50-50 criteria.
CONCLUSIONS
Linear OGTT affects the PLF and major morbidity, therein impacting the hospital stay. OGTT LI and PHRR can help predict postoperative outcome for a given extent of liver resection.
Keyword
MeSH Terms
Figure
Reference
-
1. Jarnagin WR, Gonen M, Fong Y, DeMatteo RP, Ben-Porat L, Little S, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002; 236:397–406. discussion 406-407.2. Imamura H, Seyama Y, Kokudo N, Maema A, Sugawara Y, Sano K, et al. One thousand fifty-six hepatectomies without mortality in 8 years. Arch Surg. 2003; 138:1198–1206.3. Balzan S, Belghiti J, Farges O, Ogata S, Sauvanet A, Delefosse D, et al. The “50-50 criteria” on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg. 2005; 242:824–828.4. Shoup M, Gonen M, D'Angelica M, Jarnagin WR, DeMatteo RP, Schwartz LH, et al. Volumetric analysis predicts hepatic dysfunction in patients undergoing major liver resection. J Gastrointest Surg. 2003; 7:325–330.5. Schindl MJ, Redhead DN, Fearon KC, Garden OJ, Wigmore SJ. Edinburgh Liver Surgery and Transplantation Experimental Research Group (eLISTER). The value of residual liver volume as a predictor of hepatic dysfunction and infection after major liver resection. Gut. 2005; 54:289–296.6. Poon RT, Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, et al. Improving perioperative outcome expands the role of hepatectomy in management of benign and malignant hepatobiliary diseases: analysis of 1222 consecutive patients from a prospective database. Ann Surg. 2004; 240:698–708. discussion 708-710.7. Detroz B, Sugarbaker PH, Knol JA, Petrelli N, Hughes KS. Causes of death in patients undergoing liver surgery. Cancer Treat Res. 1994; 69:241–257.8. Bolder U, Brune A, Schmidt S, Tacke J, Jauch KW, Löhlein D. Preoperative assessment of mortality risk in hepatic resection by clinical variables: a multivariate analysis. Liver Transpl Surg. 1999; 5:227–237.9. Simmonds PC, Primrose JN, Colquitt JL, Garden OJ, Poston GJ, Rees M. Surgical resection of hepatic metastases from colorectal cancer: a systematic review of published studies. Br J Cancer. 2006; 94:982–999.10. McCormack L, Capitanich P, Quiñonez E. Liver surgery in the presence of cirrhosis or steatosis: Is morbidity increased? Patient Saf Surg. 2008; 2:8.11. Nonami T, Nakao A, Kurokawa T, Inagaki H, Matsushita Y, Sakamoto J, et al. Blood loss and ICG clearance as best prognostic markers of post-hepatectomy liver failure. Hepatogastroenterology. 1999; 46:1669–1672.12. Fan ST. Methods and related drawbacks in the estimation of surgical risks in cirrhotic patients undergoing hepatectomy. Hepatogastroenterology. 2002; 49:17–20.13. Ozawa K, Ida T, Yamada T, Honjo I. Significance of glucose tolerance as prognostic sign in hepatectomized patients. Am J Surg. 1976; 131:541–546.14. Ozawa K, Ida T, Yamada T, Yamaoka Y, Takasan H. Oral glucose tolerance in patients with jaundice. Surg Gynecol Obstet. 1975; 140:582–588.15. Mari A, Pacini G, Murphy E, Ludvik B, Nolan JJ. A model-based method for assessing insulin sensitivity from the oral glucose tolerance test. Diabetes Care. 2001; 24:539–548.16. Nolan JJ, Ludvik B, Baloga J, Reichart D, Olefsky JM. Mechanisms of the kinetic defect in insulin action in obesity and NIDDM. Diabetes. 1997; 46:994–1000.17. Uchiyama K, Mori K, Tabuse K, Ueno M, Ozawa S, Nakase T, et al. Assessment of liver function for successful hepatectomy in patients with hepatocellular carcinoma with impaired hepatic function. J Hepatobiliary Pancreat Surg. 2008; 15:596–602.18. Okamoto E, Kyo A, Yamanaka N, Tanaka N, Kuwata K. Prediction of the safe limits of hepatectomy by combined volumetric and functional measurements in patients with impaired hepatic function. Surgery. 1984; 95:586–592.19. Strasberg SM. Nomenclature of hepatic anatomy and resections: a review of the Brisbane 2000 system. J Hepatobiliary Pancreat Surg. 2005; 12:351–355.20. Maeda Y, Nishida M, Takao T, Mori N, Tamesa T, Tangoku A, et al. Risk factors for postoperative liver failure after hepatectomy for hepatocellular carcinoma. Hepatogastroenterology. 2004; 51:1792–1796.21. Belghiti J, Hiramatsu K, Benoist S, Massault P, Sauvanet A, Farges O. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg. 2000; 191:38–46.22. Hwang SJ, Luo JC, Chu CW, Lai CR, Lu CL, Tsay SH, et al. Hepatic steatosis in chronic hepatitis C virus infection: prevalence and clinical correlation. J Gastroenterol Hepatol. 2001; 16:190–195.23. Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995; 22:696–699.24. Marwah S, Khan MM, Chaudhary A, Gupta S, Negi SS, Soin A, et al. Two hundred and forty-one consecutive liver resections: an experience from India. HPB (Oxford). 2007; 9:29–36.25. Garski TR, Staller BJ, Hepner G, Banka VS, Finney RA Jr. Adverse reactions after administration of indocyanine green. JAMA. 1978; 240:635.26. Speich R, Saesseli B, Hoffmann U, Neftel KA, Reichen J. Anaphylactoid reactions after indocyanine-green administration. Ann Intern Med. 1988; 109:345–346.27. Benya R, Quintana J, Brundage B. Adverse reactions to indocyanine green: a case report and a review of the literature. Cathet Cardiovasc Diagn. 1989; 17:231–233.28. Schneider PD. Preoperative assessment of liver function. Surg Clin North Am. 2004; 84:355–373.29. Stockmann M, Malinowski M, Lock JF, Seehofer D, Neuhaus P. Factors influencing the indocyanine green (ICG) test: additional impact of acute cholestasis. Hepatogastroenterology. 2009; 56:734–738.30. Irie R, Kono Y, Aoyama H, Nakatani T, Yasuda K, Ozawa K, et al. Impaired glucose tolerance related to changes in the energy metabolism of the remnant liver after major hepatic resection. J Lab Clin Med. 1983; 101:692–698.31. Nakatani T, Yasuda K, Ozawa K, Tobe T. Changes in blood glucose levels in relation to blood ketone body ratio following hypertonic glucose infusion in 70% hepatectomized rabbits. Eur Surg Res. 1984; 16:303–311.32. Yasuda K, Nakatani T, Ozawa K. Glucose intolerance and amino acid imbalance in relation to changes in blood ketone body ratio in hepatectomized rabbits. Eur Surg Res. 1986; 18:19–27.33. Uchida K, Jikko A, Yamato T, Kamiyama Y, Ozawa K. Relationship of glucose intolerance and indocyanine green clearance to respiratory enzyme levels in human cirrhotic liver. Am J Med Sci. 1985; 290:19–27.34. Mori K, Ozawa K, Yamamoto Y, Maki A, Shimahara Y, Kobayashi N, et al. Response of hepatic mitochondrial redox state to oral glucose load. Redox tolerance test as a new predictor of surgical risk in hepatectomy. Ann Surg. 1990; 211:438–446.35. Sasaya S, Yagi H, Yamaguchi M, Kigawa G, Nakano H, Midorikawa T, et al. Liver function assessed by increased rate of portal venous blood flow after oral intake of glucose. Eur J Surg. 2000; 166:112–118.36. Huo TI, Lui WY, Huang YH, Chau GY, Wu JC, Lee PC, et al. Diabetes mellitus is a risk factor for hepatic decompensation in patients with hepatocellular carcinoma undergoing resection: a longitudinal study. Am J Gastroenterol. 2003; 98:2293–2298.37. Yamanaka N, Okamoto E, Oriyama T, Fujimoto J, Furukawa K, Kawamura E, et al. A prediction scoring system to select the surgical treatment of liver cancer. Further refinement based on 10 years of use. Ann Surg. 1994; 219:342–346.38. Nagino M, Nimura Y, Hayakawa N, Kamiya J, Kondo S, Sasaki R, et al. Logistic regression and discriminant analyses of hepatic failure after liver resection for carcinoma of the biliary tract. World J Surg. 1993; 17:250–255.39. Nishida T, Tsuji S, Tsujii M, Arimitsu S, Haruna Y, Imano E, et al. Oral glucose tolerance test predicts prognosis of patients with liver cirrhosis. Am J Gastroenterol. 2006; 101:70–75.40. Wen T, Zheng G, Meng X, Chen L. Evaluation of oral glucose tolerance test in the assessment of reserved function of liver for patients with hepatocellular carcinoma. Hua Xi Yi Ke Da Xue Xue Bao. 1997; 28:197–200.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Significance of Several Hepatic Function Tests in Hepatectomized Patients
- Relationships between Insulin-like Growth Factor-I and Severity of Hepatic Dysfunction, and Glucose Tolerance Status in Patients with Liver Cirrhosis
- No title available
- Comparison of Glucose Intolerance according to Severity of Chronic Liver Diseases
- Soybean isoflavone extract improves glucose tolerance and raises the survival rate in streptozotocin-induced diabetic rats