Ann Rehabil Med.
2016 Dec;40(6):1129-1134. 10.5535/arm.2016.40.6.1129.
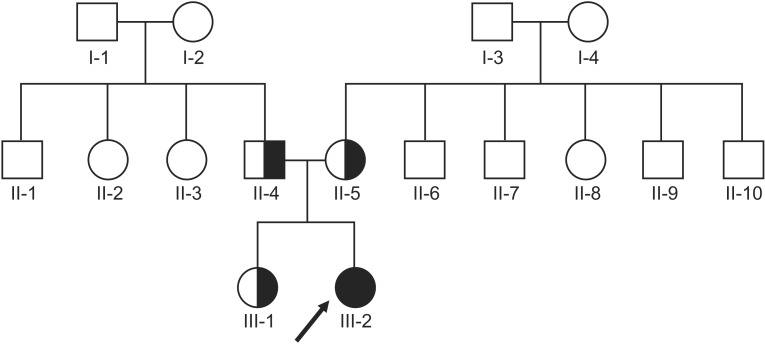
Identification of a Heterozygous SPG11 Mutation by Clinical Exome Sequencing in a Patient With Hereditary Spastic Paraplegia: A Case Report
- Affiliations
-
- 1Department of Rehabilitation, Incheon St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Incheon, Korea. dhjangmd@naver.com
- 2Department of Laboratory Medicine, Incheon St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Incheon, Korea.
- 3Green Cross Laboratories, Yongin, Korea.
- 4Green Cross Genome, Yongin, Korea.
- KMID: 2371347
- DOI: http://doi.org/10.5535/arm.2016.40.6.1129
Abstract
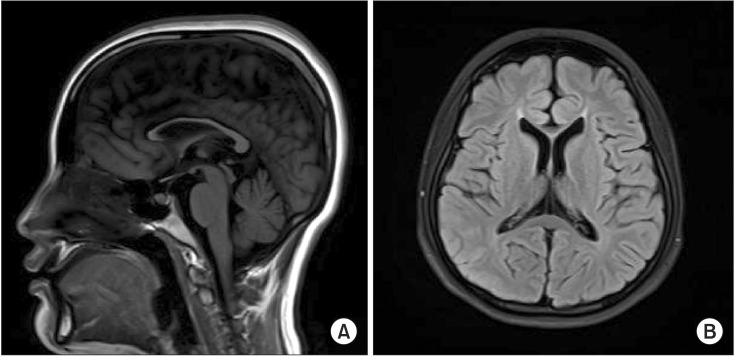
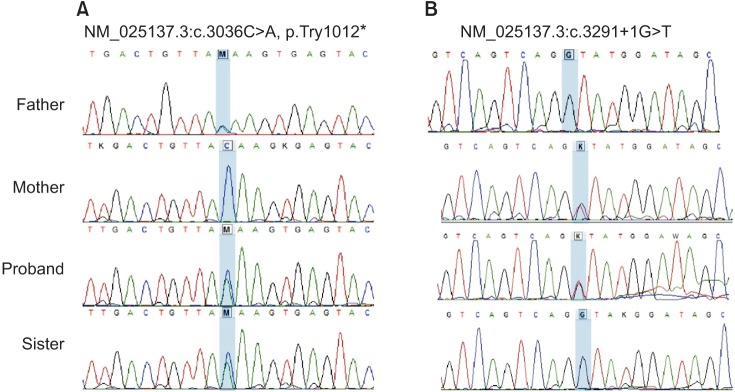
- Next-generation sequencing, such as whole-genome sequencing, whole-exome sequencing, and targeted panel sequencing have been applied for diagnosis of many genetic diseases, and are in the process of replacing the traditional methods of genetic analysis. Clinical exome sequencing (CES), which provides not only sequence variation data but also clinical interpretation, aids in reaching a final conclusion with regards to genetic diagnosis. Sequencing of genes with clinical relevance rather than whole exome sequencing might be more suitable for the diagnosis of known hereditary disease with genetic heterogeneity. Here, we present the clinical usefulness of CES for the diagnosis of hereditary spastic paraplegia (HSP). We report a case of patient who was strongly suspected of having HSP based on her clinical manifestations. HSP is one of the diseases with high genetic heterogeneity, the 72 different loci and 59 discovered genes identified so far. Therefore, traditional approach for diagnosis of HSP with genetic analysis is very challenging and time-consuming. CES with TruSight One Sequencing Panel, which enriches about 4,800 genes with clinical relevance, revealed compound heterozygous mutations in SPG11. One workflow and one procedure can provide the results of genetic analysis, and CES with enrichment of clinically relevant genes is a cost-effective and time-saving diagnostic tool for diseases with genetic heterogeneity, including HSP.
Keyword
MeSH Terms
Figure
Reference
-
1. Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977; 74:5463–5467. PMID: 271968.
Article2. Lohmann K, Klein C. Next generation sequencing and the future of genetic diagnosis. Neurotherapeutics. 2014; 11:699–707. PMID: 25052068.
Article3. Jang MA, Lee T, Lee J, Cho EH, Ki CS. Identification of a novel de novo variant in the pax3 gene in waardenburg syndrome by diagnostic exome sequencing: the first molecular diagnosis in Korea. Ann Lab Med. 2015; 35:362–365. PMID: 25932447.
Article4. Tesson C, Koht J, Stevanin G. Delving into the complexity of hereditary spastic paraplegias: how unexpected phenotypes and inheritance modes are revolutionizing their nosology. Hum Genet. 2015; 134:511–538. PMID: 25758904.
Article5. Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJ, et al. GeneReviews. Seattle: University of Washington at Seattle;c2016.6. Perez-Branguli F, Mishra HK, Prots I, Havlicek S, Kohl Z, Saul D, et al. Dysfunction of spatacsin leads to axonal pathology in SPG11-linked hereditary spastic paraplegia. Hum Mol Genet. 2014; 23:4859–4874. PMID: 24794856.7. Kim SM, Lee JS, Kim S, Kim HJ, Kim MH, Lee KM, et al. Novel compound heterozygous mutations of the SPG11 gene in Korean families with hereditary spastic paraplegia with thin corpus callosum. J Neurol. 2009; 256:1714–1718. PMID: 19513778.
Article8. Salinas S, Proukakis C, Crosby A, Warner TT. Hereditary spastic paraplegia: clinical features and pathogenetic mechanisms. Lancet Neurol. 2008; 7:1127–1138. PMID: 19007737.
Article9. Winner B, Uyanik G, Gross C, Lange M, Schulte-Mattler W, Schuierer G, et al. Clinical progression and genetic analysis in hereditary spastic paraplegia with thin corpus callosum in spastic gait gene 11 (SPG11). Arch Neurol. 2004; 61:117–121. PMID: 14732628.
Article10. Stevanin G, Durr A, Brice A. Spastic paraplegia 11. In : Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJ, editors. GeneReviews. Seattle: University of Washington at Seattle;c2013.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hereditary spastic paraplegia with thin corpus callosum due to novel homozygous mutation in SPG11 gene
- Two novel mutations in ALDH18A1 and SPG11 gene found by whole-exome sequencing in spastic paraplegia disease patients in Iran
- Thinning of the Corpus Callosum and Cerebellar Atrophy is Correlated with Phenotypic Severity in a Family with Spastic Paraplegia Type 11
- Distal Hereditary Motor Neuropathy Type V (dHMN-V) With N88S Mutation in BSCL2 Gene
- SPG11 Mutation in Hereditary Spastic Paraplegia with Thin Corpus Callosum Diagnosed by Targeted Gene Panel Sequencing