Korean J Physiol Pharmacol.
2016 Jul;20(4):399-406. 10.4196/kjpp.2016.20.4.399.
Preventive effects of imperatorin on perfluorohexanesulfonate-induced neuronal apoptosis via inhibition of intracellular calcium-mediated ERK pathway
- Affiliations
-
- 1Research and Development Division, Korea Promotion Institute for Traditional Medicine Industry, Gyeongsan 38540, Korea.
- 2Department of Pharmacology/Toxicology, School of Medicine, Catholic University of Daegu, Daegu 42472, Korea. younjul@hotmail.com
- KMID: 2371057
- DOI: http://doi.org/10.4196/kjpp.2016.20.4.399
Abstract
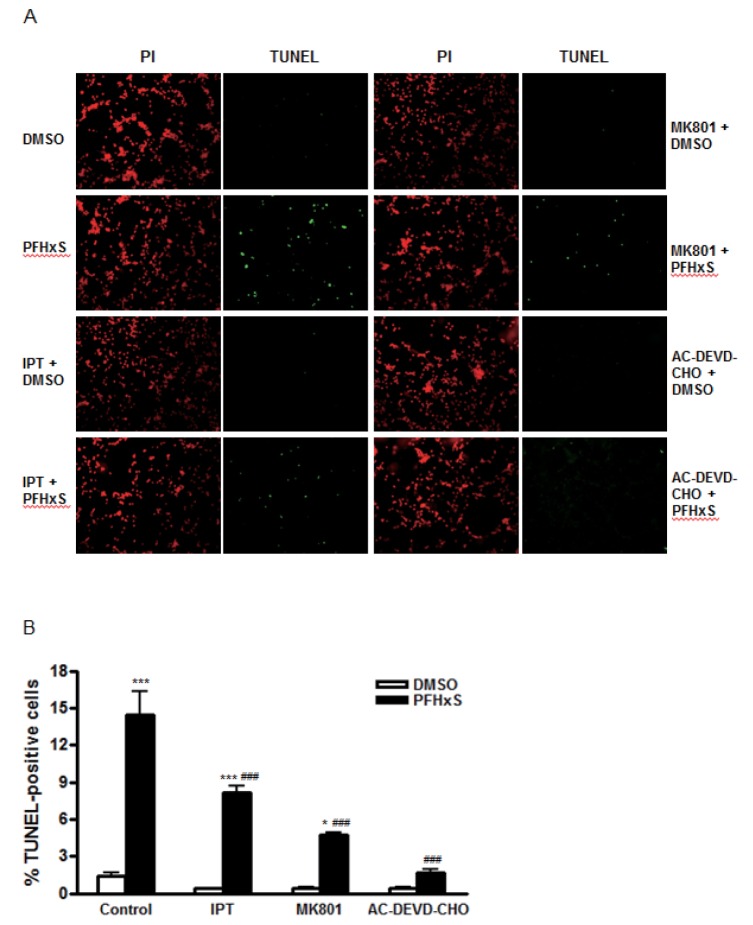
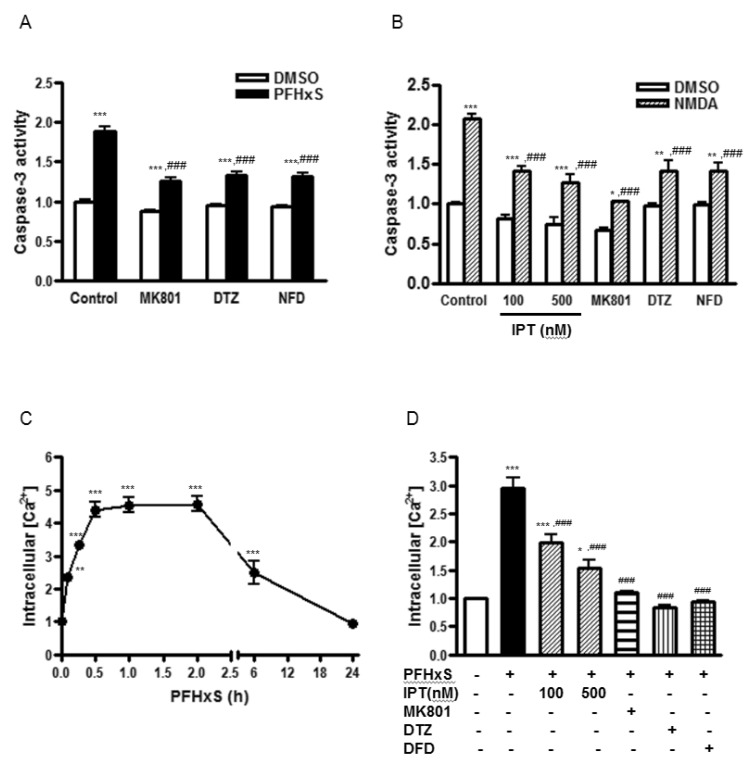
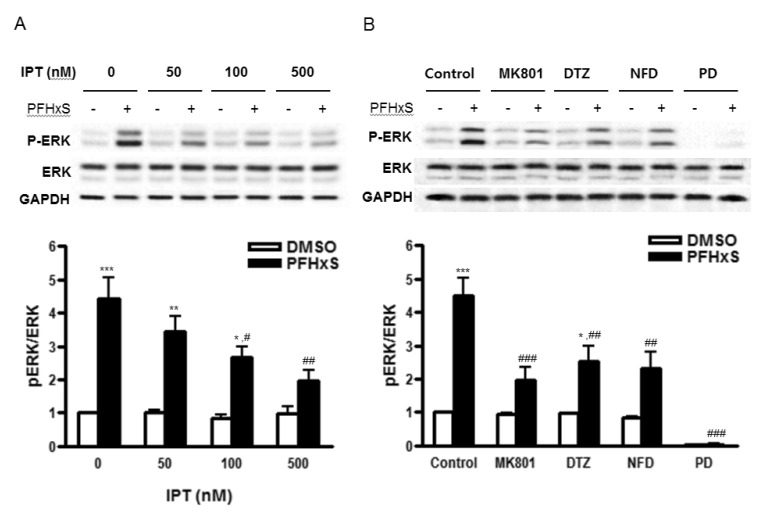
- Early life neuronal exposure to environmental toxicants has been suggested to be an important etiology of neurodegenerative disease development. Perfluorohexanesulfonate (PFHxS), one of the major perfluoroalkyl compounds, is widely distributed environmental contaminants. We have reported that PFHxS induces neuronal apoptosis via ERK-mediated pathway. Imperatorin is a furanocoumarin found in various edible plants and has a wide range of pharmacological effects including neuroprotection. In this study, the effects of imperatorin on PFHxS-induced neuronal apoptosis and the underlying mechanisms are examined using cerebellar granule cells (CGC). CGC were isolated from seven-day old rats and were grown in culture for seven days. Caspase-3 activity and TUNEL staining were used to determine neuronal apoptosis. PFHxS-induced apoptosis of CGC was significantly reduced by imperatorin and PD98059, an ERK pathway inhibitor. PFHxS induced a persistent increase in intracellular calcium, which was significantly blocked by imperatorin, NMDA receptor antagonist, MK801 and the L-type voltage-dependent calcium channel blockers, diltiazem and nifedipine. The activation of caspase-3 by PFHxS was also inhibited by MK801, diltiazem and nifedipine. PFHxS-increased ERK activation was inhibited by imperatorin, MK801, diltiazem and nifedipine. Taken together, imperatorin protects CGC against PFHxS-induced apoptosis via inhibition of NMDA receptor/intracellular calcium-mediated ERK pathway.
MeSH Terms
-
Animals
Apoptosis*
Calcium
Calcium Channel Blockers
Caspase 3
Diltiazem
Dizocilpine Maleate
In Situ Nick-End Labeling
MAP Kinase Signaling System*
N-Methylaspartate
Neurodegenerative Diseases
Neurons*
Neuroprotection
Nifedipine
Plants, Edible
Rats
Calcium
Calcium Channel Blockers
Caspase 3
Diltiazem
Dizocilpine Maleate
N-Methylaspartate
Nifedipine
Figure
Reference
-
1. Caudle WM, Guillot TS, Lazo CR, Miller GW. Industrial toxicants and Parkinson's disease. Neurotoxicology. 2012; 33:178–188. PMID: 22309908.
Article2. Trojsi F, Monsurro MR, Tedeschi G. Exposure to environmental toxicants and pathogenesis of amyotrophic lateral sclerosis: state of the art and research perspectives. Int J Mol Sci. 2013; 14:15286–15311. PMID: 23887652.3. Fox DA, Grandjean P, de Groot D, Paule MG. Developmental origins of adult diseases and neurotoxicity: epidemiological and experimental studies. Neurotoxicology. 2012; 33:810–816. PMID: 22245043.
Article4. Buck RC, Franklin J, Berger U, Conder JM, Cousins IT, de Voogt P, Jensen AA, Kannan K, Mabury SA, van Leeuwen SP. Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integr Environ Assess Manag. 2011; 7:513–541. PMID: 21793199.
Article5. Gump BB, Wu Q, Dumas AK, Kannan K. Perfluorochemical (PFC) exposure in children: associations with impaired response inhibition. Environ Sci Technol. 2011; 45:8151–8159. PMID: 21682250.
Article6. Viberg H, Lee I, Eriksson P. Adult dose-dependent behavioral and cognitive disturbances after a single neonatal PFHxS dose. Toxicology. 2013; 304:185–191. PMID: 23287389.
Article7. Olney JW. New insights and new issues in developmental neurotoxicology. Neurotoxicology. 2002; 23:659–668. PMID: 12520755.
Article8. Lee YJ, Choi SY, Yang JH. PFHxS induces apoptosis of neuronal cells via ERK1/2-mediated pathway. Chemosphere. 2014; 94:121–127. PMID: 24125707.
Article9. Dobbing J, Sands J. Quantitative growth and development of human brain. Arch Dis Child. 1973; 48:757–767. PMID: 4796010.
Article10. Bonfoco E, Leist M, Zhivotovsky B, Orrenius S, Lipton SA, Nicotera P. Cytoskeletal breakdown and apoptosis elicited by NO donors in cerebellar granule cells require NMDA receptor activation. J Neurochem. 1996; 67:2484–2493. PMID: 8931482.
Article11. Tenneti L, D'Emilia DM, Troy CM, Lipton SA. Role of caspases in N-methyl-D-aspartate-induced apoptosis in cerebrocortical neurons. J Neurochem. 1998; 71:946–959. PMID: 9721720.
Article12. Yamagishi S, Matsumoto T, Numakawa T, Yokomaku D, Adachi N, Hatanaka H, Yamada M, Shimoke K, Ikeuchi T. ERK1/2 are involved in low potassium-induced apoptotic signaling downstream of ASK1-p38 MAPK pathway in cultured cerebellar granule neurons. Brain Res. 2005; 1038:223–230. PMID: 15757638.
Article13. Vacotto M, Coso O, Fiszer de Plazas S. Programmed cell death and differential JNK, p38 and ERK response in a prenatal acute hypoxic hypoxia model. Neurochem Int. 2008; 52:857–863. PMID: 18077057.
Article14. Crowder JM, Croucher MJ, Bradford HF, Collins JF. Excitatory amino acid receptors and depolarization-induced Ca2+ influx into hippocampal slices. J Neurochem. 1987; 48:1917–1924. PMID: 2437250.15. Manev H, Favaron M, Guidotti A, Costa E. Delayed increase of Ca2+ influx elicited by glutamate: role in neuronal death. Mol Pharmacol. 1989; 36:106–112. PMID: 2568579.16. Urushitani M, Nakamizo T, Inoue R, Sawada H, Kihara T, Honda K, Akaike A, Shimohama S. N-methyl-D-aspartate receptor-mediated mitochondrial Ca2+ overload in acute excitotoxic motor neuron death: a mechanism distinct from chronic neurotoxicity after Ca2+ influx. J Neurosci Res. 2001; 63:377–387. PMID: 11223912.17. Auzmendi J, Gonzalez N, Girardi E. The NMDAR subunit NR2B expression is modified in hippocampus after repetitive seizures. Neurochem Res. 2009; 34:819–826. PMID: 18751892.
Article18. Choi DW. Glutamate receptors and the induction of excitotoxic neuronal death. Prog Brain Res. 1994; 100:47–51. PMID: 7938533.19. Hsieh MH, Gu SL, Ho SC, Pawlak CR, Lin CL, Ho YJ, Lai TJ, Wu FY. Effects of MK-801 on recognition and neurodegeneration in an MPTP-induced Parkinson's rat model. Behav Brain Res. 2012; 229:41–47. PMID: 22227506.
Article20. Mota SI, Ferreira IL, Rego AC. Dysfunctional synapse in Alzheimer's disease - a focus on NMDA receptors. Neuropharmacology. 2014; 76:16–26. PMID: 23973316.
Article21. Olivares D, Deshpande VK, Shi Y, Lahiri DK, Greig NH, Rogers JT, Huang X. N-methyl D-aspartate (NMDA) receptor antagonists and memantine treatment for Alzheimer's disease, vascular dementia and Parkinson's disease. Curr Alzheimer Res. 2012; 9:746–758. PMID: 21875407.
Article22. Fan J, Gladding CM, Wang L, Zhang LY, Kaufman AM, Milnerwood AJ, Raymond LA. P38 MAPK is involved in enhanced NMDA receptor-dependent excitotoxicity in YAC transgenic mouse model of Huntington disease. Neurobiol Dis. 2012; 45:999–1009. PMID: 22198502.
Article23. Poddar R, Paul S. Novel crosstalk between ERK MAPK and p38 MAPK leads to homocysteine-NMDA receptor-mediated neuronal cell death. J Neurochem. 2013; 124:558–570. PMID: 23176034.
Article24. Xiao L, Feng C, Chen Y. Glucocorticoid rapidly enhances NMDAevoked neurotoxicity by attenuating the NR2A-containing NMDA receptor-mediated ERK1/2 activation. Mol Endocrinol. 2010; 24:497–510. PMID: 20160127.
Article25. Lee YJ, Choi SY, Yang JH. NMDA receptor-mediated ERK 1/2 pathway is involved in PFHxS-induced apoptosis of PC12 cells. Sci Total Environ. 2014; 491-492:227–234. PMID: 24534200.
Article26. Baek NI, Ahn EM, Kim HY, Park YD. Furanocoumarins from the root of angelica dahurica. Arch Pharm Res. 2000; 23:467–470. PMID: 11059825.27. Lia HB, Chen F. Preparative isolation and purification of bergapten and imperatorin from the medicinal plant cnidium monnieri using high-speed counter-current chromatography by stepwise increasing the flow-rate of the mobile phase. J Chromatogr A. 2004; 1061:51–54. PMID: 15633744.28. Howes MJ, Perry NS, Houghton PJ. Plants with traditional uses and activities, relevant to the management of Alzheimer's disease and other cognitive disorders. Phytother Res. 2003; 17:1–18. PMID: 12557240.
Article29. Wang N, Wu L, Cao Y, Wang Y, Zhang Y. The protective activity of imperatorin in cultured neural cells exposed to hypoxia reoxygenation injury via anti-apoptosis. Fitoterapia. 2013; 90:38–43. PMID: 23856091.
Article30. Budzynska B, Boguszewska-Czubara A, Kruk-Slomka M, Skalicka-Wozniak K, Michalak A, Musik I, Biala G. Effects of imperatorin on scopolamine-induced cognitive impairment and oxidative stress in mice. Psychopharmacology (Berl). 2015; 232:931–942. PMID: 25189792.
Article31. Lili W, Yehong S, Qi Y, Yan H, Jinhui Z, Yan L, Cheng G. In vitro permeability analysis, pharmacokinetic and brain distribution study in mice of imperatorin, isoimperatorin and cnidilin in Radix Angelicae Dahuricae. Fitoterapia. 2013; 85:144–153. PMID: 23353658.
Article32. Wang Y, Wang N, Wu L, Lu W, Zhang Y. Simultaneous determination of four components in baige capsule by HPLC: application to pharmacokinetics and tissue distribution of normal and middle cerebral artery occlusion rats. Biomed Chromatogr. 2014; 28:541–547. PMID: 24122939.
Article33. Yang JH, Kodavanti PR. Possible molecular targets of halogenated aromatic hydrocarbons in neuronal cells. Biochem Biophys Res Commun. 2001; 280:1372–1377. PMID: 11162682.
Article34. Cariou R, Veyrand B, Yamada A, Berrebi A, Zalko D, Durand S, Pollono C, Marchand P, Leblanc JC, Antignac JP, Le Bizec B. Perfluoroalkyl acid (PFAA) levels and profiles in breast milk, maternal and cord serum of French women and their newborns. Environ Int. 2015; 84:71–81. PMID: 26232143.
Article35. Maisonet M, Calafat AM, Marcus M, Jaakkola JJ, Lashen H. Prenatal exposure to perfluoroalkyl acids and serum testosterone concentrations at 15 years of age in female ALSPAC study participants. Environ Health Perspect. 2015; 123:1325–1330. PMID: 26034840.
Article36. Manzano-Salgado CB, Casas M, Lopez-Espinosa MJ, Ballester F, Basterrechea M, Grimalt JO, Jimenez AM, Kraus T, Schettgen T, Sunyer J, Vrijheid M. Transfer of perfluoroalkyl substances from mother to fetus in a Spanish birth cohort. Environ Res. 2015; 142:471–478. PMID: 26257032.
Article37. Porterfield SP. Vulnerability of the developing brain to thyroid abnormalities: environmental insults to the thyroid system. Environ Health Perspect. 1994; 102(Suppl 2):125–130. PMID: 7925183.
Article38. Wood NS, Costeloe K, Gibson AT, Hennessy EM, Marlow N, Wilkinson AR. EPICure Study Group. The EPICure study: associations and antecedents of neurological and developmental disability at 30 months of age following extremely preterm birth. Arch Dis Child Fetal Neonatal Ed. 2005; 90:F134–F140. PMID: 15724037.
Article39. Aarts M, Liu Y, Liu L, Besshoh S, Arundine M, Gurd JW, Wang YT, Salter MW, Tymianski M. Treatment of ischemic brain damage by perturbing NMDA receptor-PSD-95 protein interactions. Science. 2002; 298:846–850. PMID: 12399596.40. Fagni L, Chavis P, Ango F, Bockaert J. Complex interactions between mGluRs, intracellular Ca2+ stores and ion channels in neurons. Trends Neurosci. 2000; 23:80–88. PMID: 10652549.41. Sukhareva M, Smith SV, Maric D, Barker JL. Functional properties of ryanodine receptors in hippocampal neurons change during early differentiation in culture. J Neurophysiol. 2002; 88:1077–1087. PMID: 12205130.
Article42. He JY, Zhang W, He LC, Cao YX. Imperatorin induces vasodilatation possibly via inhibiting voltage dependent calcium channel and receptor-mediated Ca2+ influx and release. Eur J Pharmacol. 2007; 573:170–175. PMID: 17662269.43. Zhang Y, Cao Y, Wang Q, Zheng L, Zhang J, He L. A potential calcium antagonist and its antihypertensive effects. Fitoterapia. 2011; 82:988–996. PMID: 21679750.
Article44. Wang SJ, Lin TY, Lu CW, Huang WJ. Osthole and imperatorin, the active constituents of cnidium monnieri (L.) cusson, facilitate glutamate release from rat hippocampal nerve terminals. Neurochem Int. 2008; 53:416–423. PMID: 18951936.
Article45. Shiflett MW, Balleine BW. Contributions of ERK signaling in the striatum to instrumental learning and performance. Behav Brain Res. 2011; 218:240–247. PMID: 21147168.
Article46. Webb IC, Coolen LM, Lehman MN. NMDA and PACAP receptor signaling interact to mediate retinal-induced scn cellular rhythmicity in the absence of light. PLoS One. 2013; 8:e76365. PMID: 24098484.
Article47. Agell N, Bachs O, Rocamora N, Villalonga P. Modulation of the Ras/Raf/MEK/ERK pathway by Ca2+, and calmodulin. Cell Signal. 2002; 14:649–654. PMID: 12020764.48. Qian W, Zhu J, Mao C, Liu J, Wang Y, Wang Q, Liu Y, Gao R, Xiao H, Wang J. Involvement of CaM-CaMKII-ERK in bisphenol A-induced Sertoli cell apoptosis. Toxicology. 2014; 324:27–34. PMID: 24905940.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Extracellular Signal-Regulated Kinase Inhibition on Oxysterol 7-Ketocholesterol-Induced Apoptosis
- Imperatorin Suppresses Degranulation and Eicosanoid Generation in Activated Bone Marrow-Derived Mast Cells
- Thrombin Induced Apoptosis through Calcium-Mediated Activation of Cytosolic Phospholipase A2 in Intestinal Myofibroblasts
- Endothelin 1 protects HN33 cells from serum deprivation-induced neuronal apoptosis through Ca2+-PKCalpha-ERK pathway
- Modulation of Dopaminergic Neuronal Excitability by Zinc through the Regulation of Calcium-related Channels