Korean J Physiol Pharmacol.
2017 Mar;21(2):241-249. 10.4196/kjpp.2017.21.2.241.
Intracellular calcium-dependent regulation of the sperm-specific calcium-activated potassium channel, hSlo3, by the BK(Ca) activator LDD175
- Affiliations
-
- 1Laboratory of Physiology, College of Veterinary Medicine, Chungnam National University, Daejeon 34134, Korea. kplee@cnu.ac.kr
- 2Department of Physiology, College of Medicine, Gachon University, Incheon 21936, Korea.
- KMID: 2371043
- DOI: http://doi.org/10.4196/kjpp.2017.21.2.241
Abstract
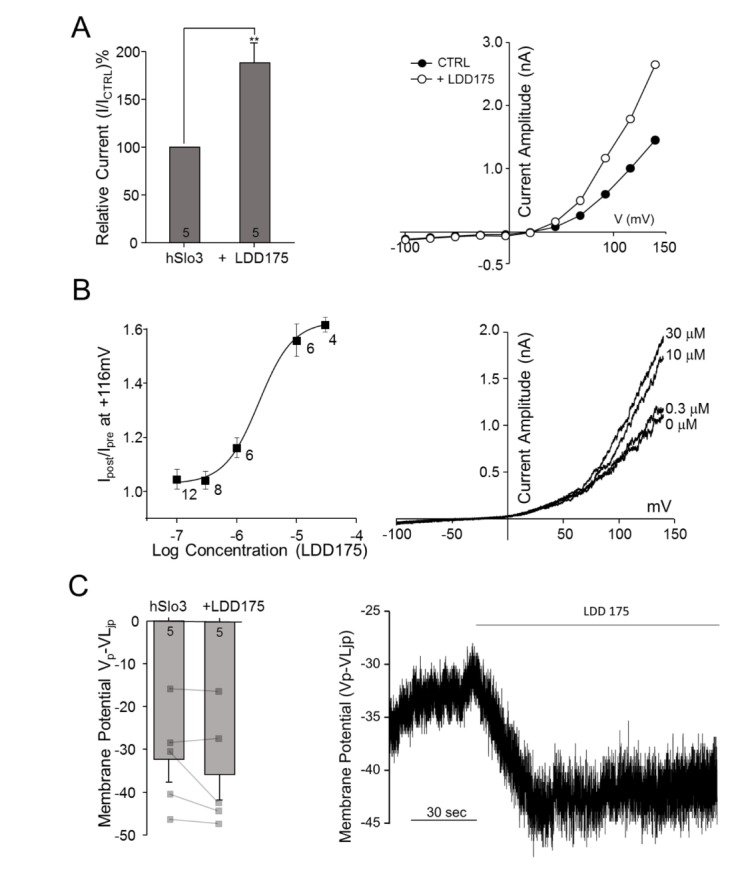
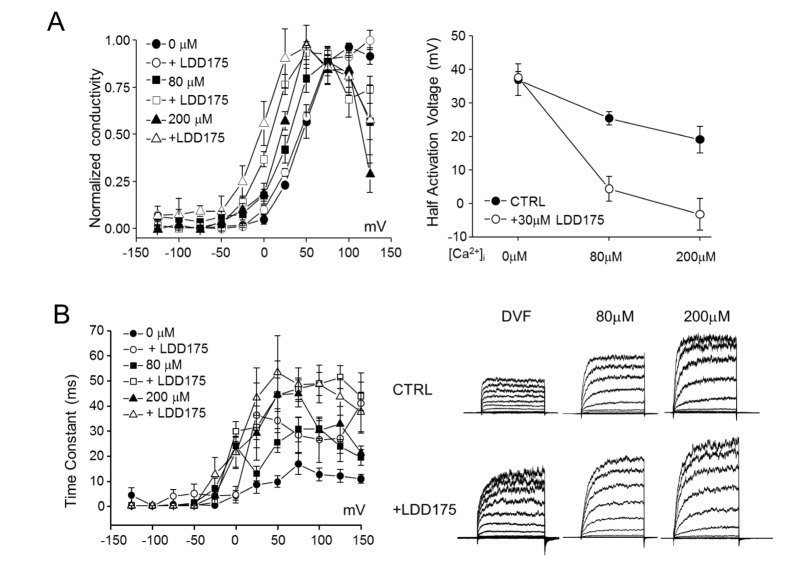
- Plasma membrane hyperpolarization associated with activation of Ca²âº-activated K⺠channels plays an important role in sperm capacitation during fertilization. Although Slo3 (slowpoke homologue 3), together with the auxiliary γ2-subunit, LRRC52 (leucine-rich-repeat-containing 52), is known to mediate the pH-sensitive, sperm-specific K⺠current KSper in mice, the molecular identity of this channel in human sperm remains controversial. In this study, we tested the classical BK(Ca) activators, NS1619 and LDD175, on human Slo3, heterologously expressed in HEK293 cells together with its functional interacting γ2 subunit, hLRRC52. As previously reported, Slo3 K⺠current was unaffected by iberiotoxin or 4-aminopyridine, but was inhibited by ~50% by 20 mM TEA. Extracellular alkalinization potentiated hSlo3 K⺠current, and internal alkalinization and Ca²âº elevation induced a leftward shift its activation voltage. NS1619, which acts intracellularly to modulate hSlo1 gating, attenuated hSlo3 K⺠currents, whereas LDD175 increased this current and induced membrane potential hyperpolarization. LDD175-induced potentiation was not associated with a change in the half-activation voltage at different intracellular pHs (pH 7.3 and pH 8.0) in the absence of intracellular Ca²âº. In contrast, elevation of intracellular Ca²âº dramatically enhanced the LDD175-induced leftward shift in the half-activation potential of hSlo3. Therefore, the mechanism of action does not involve pH-dependent modulation of hSlo3 gating; instead, LDD175 may modulate Ca²âº-dependent activation of hSlo3. Thus, LDD175 potentially activates native KSper and may induce membrane hyperpolarization-associated hyperactivation in human sperm.
Keyword
MeSH Terms
Figure
Reference
-
1. Kirichok Y, Navarro B, Clapham DE. Whole-cell patch-clamp measurements of spermatozoa reveal an alkaline-activated Ca2+ channel. Nature. 2006; 439:737–740. PMID: 16467839.2. Santi CM, Martínez-López P, de la Vega-Beltrán JL, Butler A, Alisio A, Darszon A, Salkoff L. The SLO3 sperm-specific potassium channel plays a vital role in male fertility. FEBS Lett. 2010; 584:1041–1046. PMID: 20138882.
Article3. Zeng XH, Yang C, Kim ST, Lingle CJ, Xia XM. Deletion of the Slo3 gene abolishes alkalization-activated K+ current in mouse spermatozoa. Proc Natl Acad Sci U S A. 2011; 108:5879–5884. PMID: 21427226.4. Yang C, Zeng XH, Zhou Y, Xia XM, Lingle CJ. LRRC52 (leucine-rich-repeat-containing protein 52), a testis-specific auxiliary subunit of the alkalization-activated Slo3 channel. Proc Natl Acad Sci U S A. 2011; 108:19419–19424. PMID: 22084117.
Article5. Mannowetz N, Naidoo NM, Choo SA, Smith JF, Lishko PV. Slo1 is the principal potassium channel of human spermatozoa. Elife. 2013; 2:e01009. PMID: 24137539.
Article6. Brenker C, Zhou Y, Müller A, Echeverry FA, Trötschel C, Poetsch A, Xia XM, Bönigk W, Lingle CJ, Kaupp UB, Strünker T. The Ca2+-activated K+ current of human sperm is mediated by Slo3. Elife. 2014; 3:e01438. PMID: 24670955.7. Leonetti MD, Yuan P, Hsiung Y, Mackinnon R. Functional and structural analysis of the human SLO3 pH- and voltage-gated K+ channel. Proc Natl Acad Sci U S A. 2012; 109:19274–19279. PMID: 23129643.8. Zeng XH, Yang C, Xia XM, Liu M, Lingle CJ. SLO3 auxiliary subunit LRRC52 controls gating of sperm KSPER currents and is critical for normal fertility. Proc Natl Acad Sci U S A. 2015; 112:2599–2604. PMID: 25675513.
Article10. Okabe M. The cell biology of mammalian fertilization. Development. 2013; 140:4471–4479. PMID: 24194470.
Article11. Tang QY, Zhang Z, Xia XM, Lingle CJ. Block of mouse Slo1 and Slo3 K+ channels by CTX, IbTX, TEA, 4-AP and quinidine. Channels (Austin). 2010; 4:22–41. PMID: 19934650.12. Mansell SA, Publicover SJ, Barratt CL, Wilson SM. Patch clamp studies of human sperm under physiological ionic conditions reveal three functionally and pharmacologically distinct cation channels. Mol Hum Reprod. 2014; 20:392–408. PMID: 24442342.
Article13. Gribkoff VK, Lum-Ragan JT, Boissard CG, Post-Munson DJ, Meanwell NA, Starrett JE Jr, Kozlowski ES, Romine JL, Trojnacki JT, Mckay MC, Zhong J, Dworetzky SI. Effects of channel modulators on cloned large-conductance calcium-activated potassium channels. Mol Pharmacol. 1996; 50:206–217. PMID: 8700114.14. Holland M, Langton PD, Standen NB, Boyle JP. Effects of the BKCa channel activator, NS1619, on rat cerebral artery smooth muscle. Br J Pharmacol. 1996; 117:119–129. PMID: 8825352.15. Bentzen BH, Nardi A, Calloe K, Madsen LS, Olesen SP, Grunnet M. The small molecule NS11021 is a potent and specific activator of Ca2+-activated big-conductance K+ channels. Mol Pharmacol. 2007; 72:1033–1044. PMID: 17636045.16. Gormemis AE, Ha TS, Im I, Jung KY, Lee JY, Park CS, Kim YC. Benzofuroindole analogues as potent BK(Ca) channel openers. Chembiochem. 2005; 6:1745–1748. PMID: 16149102.
Article17. dela Peña IC, Yoon SY, Kim SM, Lee GS, Ryu JH, Park CS, Kim YC, Cheong JH. Bladder-relaxant properties of the novel benzofuroindole analogue LDD175. Pharmacology. 2009; 83:367–378. PMID: 19451752.
Article18. Dela Peña IC, Yoon SY, Kim SM, Lee GS, Park CS, Kim YC, Cheong JH. Inhibition of intestinal motility by the putative BK(Ca) channel opener LDD175. Arch Pharm Res. 2009; 32:413–420. PMID: 19387586.
Article19. Sung HH, Choo SH, Han DH, Chae MR, Kang SJ, Park CS, So I, Park JK, Lee SW. Effect of the novel BKCa channel opener LDD175 on the modulation of corporal smooth muscle tone. J Sex Med. 2015; 12:29–38. PMID: 25385091.20. Ha TS, Lim HH, Lee GE, Kim YC, Park CS. Electrophysiological characterization of benzofuroindole-induced potentiation of large-conductance Ca2+-activated K+ channels. Mol Pharmacol. 2006; 69:1007–1014. PMID: 16332986.21. Lee BC, Lim HH, Kim S, Youn HS, Lee Y, Kim YC, Eom SH, Lee KW, Park CS. Localization of a site of action for benzofuroindole-induced potentiation of BKCa channels. Mol Pharmacol. 2012; 82:143–155. PMID: 22547262.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Spike Frequency Adaptation in Neurons of the Central Nervous System
- Effects of Fluoxetine on Potassium Channels in Cat Gastric Smooth Muscle Cells
- Altered Expressions of Calcium-Activated Potassium Channel and Connexin in Bladder Mucosae of Stress Urinary Incontinence Patients with Overactive Bladder Symptoms
- Effects of Phytoestrogen on Potassium Channel Activities of Smooth Muscle Cells of Rabbit Seminal Vesicle
- Physiological Roles and Properties of Ion Channels in Corporal Smooth Muscle