Ann Lab Med.
2017 May;37(3):195-203. 10.3343/alm.2017.37.3.195.
Dysregulation of Telomere Lengths and Telomerase Activity in Myelodysplastic Syndrome
- Affiliations
-
- 1Department of Laboratory Medicine, Seoul National University College of Medicine, Seoul, Korea. sangmee1@snu.ac.kr soonlee@snu.ac.kr
- 2Department of Laboratory Medicine, Korea University Anam Hospital, Seoul, Korea.
- 3Cytogenetics Team, Seegene Medical Foundation, Seoul, Korea.
- 4Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea.
- 5Department of Laboratory Medicine, Seoul National University Bundang Hospital, Seongnam, Korea.
- KMID: 2369744
- DOI: http://doi.org/10.3343/alm.2017.37.3.195
Abstract
- BACKGROUND
Telomere shortening is thought to be involved in the pathophysiology of myeloid malignancies, but telomere lengths (TL) during interphase and metaphase in hematopoietic malignancies have not been analyzed. We aimed to assess the TLs of interphase and metaphase cells of MDS and telomerase activity (TA) and to find out prognostic significances of TL and TA.
METHODS
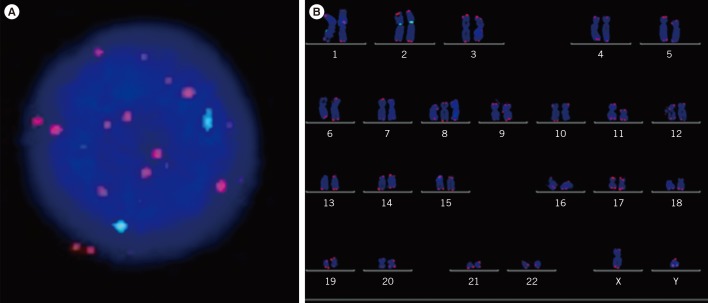
The prognostic significance of TA by quantitative PCR and TL by quantitative fluorescence in situ hybridization (QFISH) of interphase nuclei and metaphase chromosome arms of bone marrow cells from patients with MDS were evaluated.
RESULTS
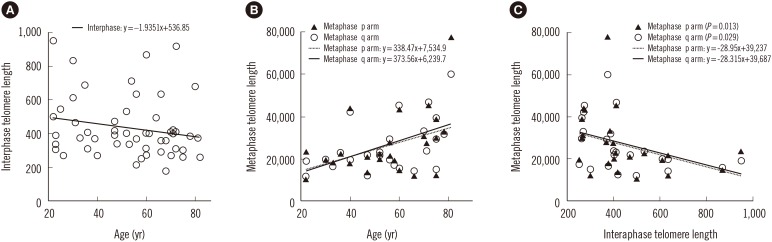
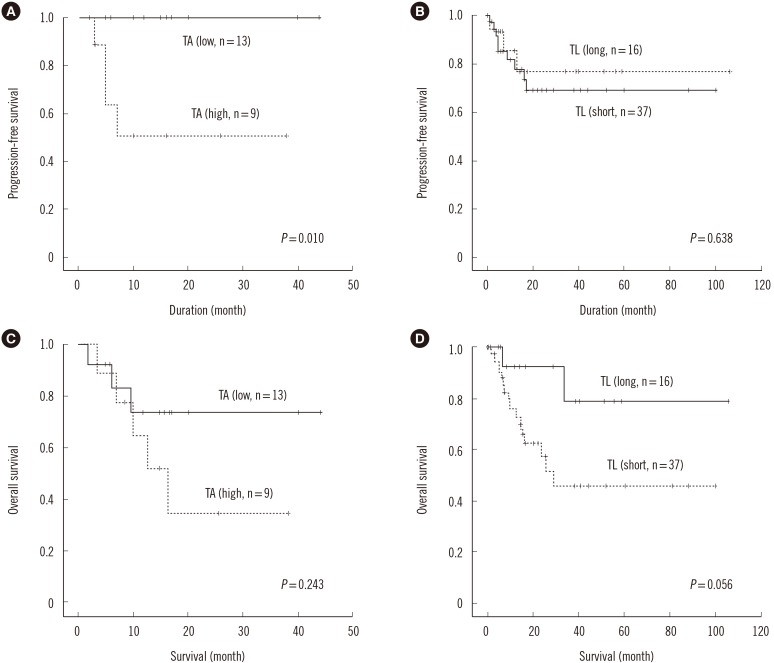
MDS patients had shorter interphase TL than normal healthy donors (P<0.001). Average interphase and metaphase TL were inversely correlated (P=0.013, p arm; P=0.029, q arm), but there was no statistically significant correlation between TA and TL (P=0.258). The progression free survival was significantly shorter in patients with high TA, but the overall survival was not different according to average TA or interphase TL groups. Multivariable Cox analysis showed that old age, higher International Prognostic Scoring System (IPSS) subtypes, transformation to AML, no history of hematopoietic stem cell transplantation and short average interphase TL (<433 TL) as independent prognostic factors for poorer survival (P=0.003, 0.001, 0.005, 0.005, and 0.013, respectively).
CONCLUSIONS
The lack of correlation between age and TL, TA, and TL, and the inverse relationship between TL and TA in MDS patients reflect the dysregulation of telomere status and proliferation. As a prognostic marker for leukemia progression, TA may be considered, and since interphase TL has the advantage of automated measurement by QFISH, it may be used as a prognostic marker for survival in MDS.
Keyword
MeSH Terms
Figure
Reference
-
1. Greider CW. Telomere length regulation. Annu Rev Biochem. 1996; 65:337–365. PMID: 8811183.2. Calado RT, Young NS. Telomere diseases. N Engl J Med. 2009; 361:2353–2365. PMID: 20007561.3. McClintock B. The behavior in successive nuclear divisions of a chromosome broken at meiosis. Proc Natl Acad Sci U S A. 1939; 25:405–416. PMID: 16577924.4. Blasco MA, Lee HW, Hande MP, Samper E, Lansdorp PM, DePinho RA, et al. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell. 1997; 91:25–34. PMID: 9335332.5. Mathieu N, Pirzio L, Freulet-Marrière MA, Desmaze C, Sabatier L. Telomeres and chromosomal instability. Cell Mol Life Sci. 2004; 61:641–656. PMID: 15052408.6. Artandi SE, Chang S, Lee SL, Alson S, Gottlieb GJ, Chin L, et al. Telomere dysfunction promotes non-reciprocal translocations and epithelial cancers in mice. Nature. 2000; 406:641–645. PMID: 10949306.7. Gürkan E, Tanrıverdi K, Başlamışlı F. Telomerase activity in myelodysplastic syndromes. Leuk Res. 2005; 29:1131–1139. PMID: 16111531.8. Swerdllow SH, Campo E, editors. WHO classification of tumours of haematopoietic and lymphoid tissues. France: IARC Press;2008.9. Mittelman M, Oster HS, Hoffman M, Neumann D. The lower risk MDS patient at risk of rapid progression. Leuk Res. 2010; 34:1551–1555. PMID: 20573398.10. Pfeilstöcker M, Tuechler H, Sanz G, Schanz J, Garcia-Manero G, Solé F, et al. Time-dependent changes in mortality and transformation risk in MDS. Blood. 2016; 128:902–910. PMID: 27335276.11. Lange K, Holm L, Vang Nielsen K, Hahn A, Hofmann W, Kreipe H, et al. Telomere shortening and chromosomal instability in myelodysplastic syndromes. Genes Chromosomes Cancer. 2010; 49:260–269. PMID: 19998444.12. Sashida G, Ohyashiki JH, Nakajima A, Sumi M, Kawakubo K, Tauchi T, et al. Telomere dynamics in myelodysplastic syndrome determined by telomere measurement of marrow metaphases. Clin Cancer Res. 2003; 9:1489–1496. PMID: 12684424.13. Ohyashiki K, Iwama H, Yahata N, Tauchi T, Kawakubo K, Shimamoto T, et al. Telomere dynamics in myelodysplastic syndromes and acute leukemic transformation. Leuk Lymphoma. 2001; 42:291–299. PMID: 11699393.14. Rigolin GM, Porta MD, Bugli AM, Castagnari B, Mauro E, Bragotti LZ, et al. Flow cytometric detection of accelerated telomere shortening in myelodysplastic syndromes: correlations with aetiological and clinical–biological findings. Eur J Haematol. 2004; 73:351–358. PMID: 15458514.15. Ohshima K, Karube K, Shimazaki K, Kamma H, Suzumiya J, Hamasaki M, et al. Imbalance between apoptosis and telomerase activity in myelodysplastic syndromes: possible role in ineffective hemopoiesis. Leuk Lymphoma. 2003; 44:1339–1346. PMID: 12952227.16. Göhring G, Lange K, Hofmann W, Nielsen KV, Hellström-Lindberg E, Roy L, et al. Telomere shortening, clonal evolution and disease progression in myelodysplastic syndrome patients with 5q deletion treated with lenalidomide. Leukemia. 2012; 26:356–358. PMID: 21799512.17. Sieglová Z, Žilovcová S, Čermák J, Řhová H, Březinová D, Dvořáková R, et al. Dynamics of telomere erosion and its association with genome instability in myelodysplastic syndromes (MDS) and acute myelogenous leukemia arising from MDS: a marker of disease prognosis? Leuk Res. 2004; 28:1013–1021. PMID: 15289012.18. Ohyashiki JH, Iwama H, Yahata N, Ando K, Hayashi S, Shay JW, et al. Telomere stability is frequently impaired in high-risk groups of patients with myelodysplastic syndromes. Clin Cancer Res. 1999; 5:1155–1160. PMID: 10353751.19. Martens UM, Zijlmans JM, Poon SS, Dragowska W, Yui J, Chavez EA, et al. Short telomeres on human chromosome 17p. Nat Genet. 1998; 18:76–80. PMID: 9425906.20. Lansdorp PM, Verwoerd NP, van de Rijke FM, Dragowska V, Little MT, Dirks RW, et al. Heterogeneity in telomere length of human chromosomes. Hum Mol Genet. 1996; 5:685–691. PMID: 8733138.21. Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997; 89:2079–2088. PMID: 9058730.22. In : Czepulkowski B, Bhatt B, editors. Basic techniques for the preparation and analysis of chromosomes from bone marrow and leukaemic blood. Human cytogenetics: malignancy and acquired abnormalities. 3rd ed. Oxford: Oxford University Press;2001. p. 1–26.23. Shaffer LG, McGowan-Jordan J, editors. ISCN 2013: an international system for human cytogenetic nomenclature. Basel: Karger Medical and Scientific Publishers;2013.24. Perner S, Brüderlein S, Hasel C, Waibel I, Holdenried A, Ciloglu N, et al. Quantifying telomere lengths of human individual chromosome arms by centromere-calibrated fluorescence in situ hybridization and digital imaging. Am J Pathol. 2003; 163:1751–1756. PMID: 14578175.25. Narath R, Lörch T, Greulich-Bode KM, Boukamp P, Ambros PF. Automatic telomere length measurements in interphase nuclei by IQ-FISH. Cytometry A. 2005; 68:113–120. PMID: 16228977.26. Ohyashiki JH, Sashida G, Tauchi T, Ohyashiki K. Telomeres and telomerase in hematologic neoplasia. Oncogene. 2002; 21:680–687. PMID: 11850796.27. Boultwood J, Fidler C, Kusec R, Rack K, Elliott PJ, Atoyebi O, et al. Telomere length in myelodysplastic syndromes. Am J Hematol. 1997; 56:266–271. PMID: 9395190.28. Mayer S, Brüderlein S, Perner S, Waibel I, Holdenried A, Ciloglu N, et al. Sex-specific telomere length profiles and age-dependent erosion dynamics of individual chromosome arms in humans. Cytogenet Genome Res. 2006; 112:194–201. PMID: 16484772.29. Xu D, Gruber A, Peterson C, Pisa P. Telomerase activity and the expression of telomerase components in acute myelogenous leukaemia. Br J Haematol. 1998; 102:1367–1375. PMID: 9753073.30. Wang Y, Fang M, Sun X, Sun J. Telomerase activity and telomere length in acute leukemia: correlations with disease progression, subtypes and overall survival. Int J Lab Hematol. 2010; 32:230–238. PMID: 19614710.