Immune Netw.
2017 Feb;17(1):68-76. 10.4110/in.2017.17.1.68.
Emerging Roles of Lymphatic Vasculature in Immunity
- Affiliations
-
- 1Department of Pathology and Immunology, Washington University in St. Louis, MO 63110, USA. kkim@path.wustl.edu
- 2Center for Vascular Research, Institute of Basic Science, Daejeon 34141, Korea. jhsong@ibs.re.kr
- KMID: 2368978
- DOI: http://doi.org/10.4110/in.2017.17.1.68
Abstract
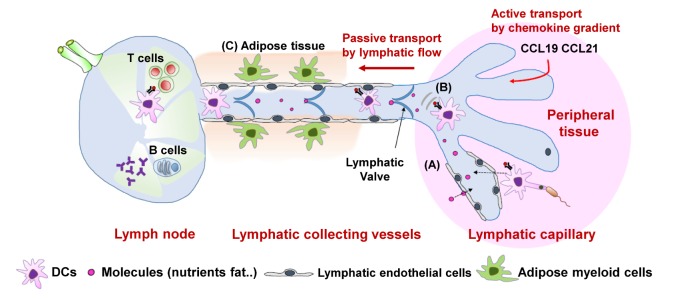
- The lymphatic vasculature has been regarded as a passive conduit for interstitial fluid and responsible for the absorption of macromolecules such as proteins or lipids and transport of nutrients from food. However, emerging data show that the lymphatic vasculature system plays an important role in immune modulation. One of its major roles is to coordinate antigen transport and immune-cell trafficking from peripheral tissues to secondary lymphoid organs, lymph nodes. This perspective was recently updated with the notion that the interaction between lymphatic endothelial cells and leukocytes controls the immune-cell migration and immune responses by regulating lymphatic flow and various secreted molecules such as chemokines and cytokines. In this review, we introduce the lymphatic vasculature networks and genetic transgenic models for research on the lymphatic vasculature system. Next, we discuss the contribution of lymphatic endothelial cells to the control of immune-cell trafficking and to maintenance of peripheral tolerance. Finally, the physiological roles and features of the lymphatic vasculature system are further discussed regarding inflammation-induced lymphangiogenesis in a pathological condition, especially in mucosal tissues such as the gastrointestinal tract and respiratory tract.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Novel Recipient Site for Aesthetic Inset of Vascularized Lymph Node Transfer: Preliminary Report
Chang Ryul Yi, Min Suk Park, Hyoung-joon Seo, Seong Hwan Bae, Jin A Yoon, Yong Chan Bae, Joo Hyoung Kim
Arch Hand Microsurg. 2021;26(2):100-108. doi: 10.12790/ahm.20.0071.
Reference
-
1. Worbs T, Hammerschmidt SI, Forster R. Dendritic cell migration in health and disease. Nat Rev Immunol. 2017; 17:30–48. PMID: 27890914.
Article2. Randolph GJ, Ivanov S, Zinselmeyer BH, Scallan JP. The lymphatic system: Integral roles in immunity. Annu Rev Immunol. 2016; DOI: 10.1146/annurevimmunol-041015-055354.
Article3. Randolph GJ, Miller NE. Lymphatic transport of high-density lipoproteins and chylomicrons. J Clin Invest. 2014; 3:929–935.
Article4. Swartz MA, Hubbell JA, Reddy ST. Lymphatic drainage function and its immunological implications: from dendritic cell homing to vaccine design. Semin Immunol. 2008; 20:147–156. PMID: 18201895.
Article5. Yang Y, Garcia-Verdugo JM, Soriano-Navarro M, Srinivasan RS, Scallan JP, Singh MK, Epstein JA, Oliver G. Lymphatic endothelial progenitors bud from the cardinal vein and intersomitic vessels in mammalian embryos. Blood. 2012; 120:2340–2348. PMID: 22859612.
Article6. Schulte-Merker S, Sabine A, Petrova TV. Lymphatic vascular morphogenesis in development, physiology, and disease. J Cell Biol. 2011; 193:607–618. PMID: 21576390.
Article7. Choi I, Chung HK, Ramu S, Lee HN, Kim KE, Lee S, Yoo J, Choi D, Lee YS, Aguilar B, Hong YK. Visualization of lymphatic vessels by Prox1-promoter directed GFP reporter in a bacterial artificial chromosome-based transgenic mouse. Blood. 2011; 117:362–365. PMID: 20962325.
Article8. Nossal GJ, Abbot A, Mitchell J. Antigens in immunity. J Exp Med. 1968; 127:263–275. PMID: 5635379.
Article9. Junt T, Moseman EA, Iannacone M, Massberg S, Lang PA, Boes M, Fink K, Henrickson SE, Shayakhmetov DM, Di Paolo NC, van RN, Mempel TR, Whelan SP, von Andrian UH. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature. 2007; 450:110–114. PMID: 17934446.
Article10. Lammermann T, Bader BL, Monkley SJ, Worbs T, Wedlich-Soldner R, Hirsch K, Keller M, Forster R, Critchley DR, Fassler R, Sixt M. Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature. 2008; 453:51–55. PMID: 18451854.
Article11. Russo E, Teijeira A, Vaahtomeri K, Willrodt AH, Bloch JS, Nitschke M, Santambrogio L, Kerjaschki D, Sixt M, Halin C. Intralymphatic CCL21 promotes tissue egress of dendritic cells through afferent lymphatic vessels. Cell Rep. 2016; 14:1723–1734. PMID: 26876174.
Article12. Ivanov S, Scallan JP, Kim KW, Werth K, Johnson MW, Saunders BT, Wang PL, Kuan EL, Straub AC, Ouhachi M, Weinstein EG, Williams JW, Briseno C, Colonna M, Isakson BE, Gautier EL, Forster R, Davis MJ, Zinselmeyer BH, Randolph GJ. CCR7 and IRF4-dependent dendritic cells regulate lymphatic collecting vessel permeability. J Clin Invest. 2016; 126:1581–1591. PMID: 26999610.
Article13. Kuan EL, Ivanov S, Bridenbaugh EA, Victora G, Wang W, Childs EW, Platt AM, Jakubzick CV, Mason RJ, Gashev AA, Nussenzweig M, Swartz MA, Dustin ML, Zawieja DC, Randolph GJ. Collecting lymphatic vessel permeability facilitates adipose tissue inflammation and distribution of antigen to lymph node-homing adipose tissue dendritic cells. J Immunol. 2015; 194:5200–5210. PMID: 25917096.
Article14. Liao S, Cheng G, Conner DA, Huang Y, Kucherlapati RS, Munn LL, Ruddle NH, Jain RK, Fukumura D, Padera TP. Impaired lymphatic contraction associated with immunosuppression. Proc Natl Acad Sci U S A. 2016; 113:E5992. PMID: 27647914.
Article15. Malhotra D, Fletcher AL, Astarita J, Lukacs-Kornek V, Tayalia P, Gonzalez SF, Elpek KG, Chang SK, Knoblich K, Hemler ME, Brenner MB, Carroll MC, Mooney DJ, Turley SJ. Transcriptional profiling of stroma from inflamed and resting lymph nodes defines immunological hallmarks. Nat Immunol. 2012; 13:499–510. PMID: 22466668.
Article16. Lukacs-Kornek V, Malhotra D, Fletcher AL, Acton SE, Elpek KG, Tayalia P, Collier AR, Turley SJ. Regulated release of nitric oxide by nonhematopoietic stroma controls expansion of the activated T cell pool in lymph nodes. Nat Immunol. 2011; 12:1096–1104. PMID: 21926986.
Article17. Podgrabinska S, Kamalu O, Mayer L, Shimaoka M, Snoeck H, Randolph GJ, Skobe M. Inflamed lymphatic endothelium suppresses dendritic cell maturation and function via Mac-1/ICAM-1-dependent mechanism. J Immunol. 2009; 183:1767–1779. PMID: 19587009.
Article18. Cohen JN, Guidi CJ, Tewalt EF, Qiao H, Rouhani SJ, Ruddell A, Farr AG, Tung KS, Engelhard VH. Lymph node-resident lymphatic endothelial cells mediate peripheral tolerance via Aire-independent direct antigen presentation. J Exp Med. 2010; 207:681–688. PMID: 20308365.
Article19. Tewalt EF, Cohen JN, Rouhani SJ, Guidi CJ, Qiao H, Fahl SP, Conaway MR, Bender TP, Tung KS, Vella AT, Adler AJ, Chen L, Engelhard VH. Lymphatic endothelial cells induce tolerance via PD-L1 and lack of costimulation leading to high-level PD-1 expression on CD8 T cells. Blood. 2012; 120:4772–4782. PMID: 22993390.
Article20. Hirosue S, Vokali E, Raghavan VR, Rincon-Restrepo M, Lund AW, Corthesy-Henrioud P, Capotosti F, Halin WC, Hugues S, Swartz MA. Steady-state antigen scavenging, cross-presentation, and CD8+ T cell priming: a new role for lymphatic endothelial cells. J Immunol. 2014; 192:5002–5011. PMID: 24795456.21. Lund AW, Duraes FV, Hirosue S, Raghavan VR, Nembrini C, Thomas SN, Issa A, Hugues S, Swartz MA. VEGF-C promotes immune tolerance in B16 melanomas and cross-presentation of tumor antigen by lymph node lymphatics. Cell Rep. 2012; 1:191–199. PMID: 22832193.
Article22. Alitalo K. The lymphatic vasculature in disease. Nat Med. 2011; 17:1371–1380. PMID: 22064427.
Article23. Zgraggen S, Ochsenbein AM, Detmar M. An important role of blood and lymphatic vessels in inflammation and allergy. J Allergy (Cairo). 2013; 2013:672381. PMID: 23431319.24. Randolph GJ, Angeli V, Swartz MA. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat Rev Immunol. 2005; 5:617–628. PMID: 16056255.
Article25. Kang S, Lee SP, Kim KE, Kim HZ, Memet S, Koh GY. Toll-like receptor 4 in lymphatic endothelial cells contributes to LPS-induced lymphangiogenesis by chemotactic recruitment of macrophages. Blood. 2009; 113:2605–2613. PMID: 19098273.
Article26. Kataru RP, Jung K, Jang C, Yang H, Schwendener RA, Baik JE, Han SH, Alitalo K, Koh GY. Critical role of CD11b+ macrophages and VEGF in inflammatory lymphangiogenesis, antigen clearance, and inflammation resolution. Blood. 2009; 113:5650–5659. PMID: 19346498.27. Ridner SH. Pathophysiology of lymphedema. Semin Oncol Nurs. 2013; 29:4–11. PMID: 23375061.
Article28. Hong YK, Lange-Asschenfeldt B, Velasco P, Hirakawa S, Kunstfeld R, Brown LF, Bohlen P, Senger DR, Detmar M. VEGFA promotes tissue repair associated lymphatic vessel formation via VEGFR2 and the α1β1 and α2β1 integrins. FASEB J. 2004; 18:1111–1113. PMID: 15132990.29. Kataru RP, Kim H, Jang C, Choi DK, Koh BI, Kim M, Gollamudi S, Kim YK, Lee SH, Koh GY. T lymphocytes negatively regulate lymph node lymphatic vessel formation. Immunity. 2011; 34:96–107. PMID: 21256057.
Article30. Garrafa E, Imberti L, Tiberio G, Prandini A, Giulini SM, Caimi L. Heterogeneous expression of toll-like receptors in lymphatic endothelial cells derived from different tissues. Immunol Cell Biol. 2011; 89:475–481. PMID: 20921966.
Article31. Saban MR, Memet S, Jackson DG, Ash J, Roig AA, Israel A, Saban R. Visualization of lymphatic vessels through NF-kappaB activity. Blood. 2004; 104:3228–3230. PMID: 15271802.32. Kang S, Lee SP, Kim KE, Kim HZ, Memet S, Koh GY. Toll-like receptor 4 in lymphatic endothelial cells contributes to LPS-induced lymphangiogenesis by chemotactic recruitment of macrophages. Blood. 2009; 113:2605–2613. PMID: 19098273.
Article33. Choe K, Jang JY, Park I, Kim Y, Ahn S, Park DY, Hong YK, Alitalo K, Koh GY, Kim P. Intravital imaging of intestinal lacteals unveils lipid drainage through contractility. J Clin Invest. 2015; 125:4042–4052. PMID: 26436648.
Article34. Jang JY, Koh YJ, Lee SH, Lee J, Kim KH, Kim D, Koh GY, Yoo OJ. Conditional ablation of LYVE-1+ cells unveils defensive roles of lymphatic vessels in intestine and lymph nodes. Blood. 2013; 122:2151–2161. PMID: 23836558.35. Miller MJ, McDole JR, Newberry RD. Microanatomy of the intestinal lymphatic system. Ann N Y Acad Sci. 2010; 1207(Suppl 1):E21–E28. PMID: 20961303.
Article36. Kaiserling E, Krober S, Geleff S. Lymphatic vessels in the colonic mucosa in ulcerative colitis. Lymphology. 2003; 36:52–61. PMID: 12926829.37. Cueni LN, Detmar M. The lymphatic system in health and disease. Lymphat Res Biol. 2008; 6:109–122. PMID: 19093783.
Article38. Backhed F, Crawford PA, O'Donnell D, Gordon JI. Postnatal lymphatic partitioning from the blood vasculature in the small intestine requires fasting-induced adipose factor. Proc Natl Acad Sci U S A. 2007; 104:606–611. PMID: 17202268.
Article39. Baluk P, Fuxe J, Hashizume H, Romano T, Lashnits E, Butz S, Vestweber D, Corada M, Molendini C, Dejana E, McDonald DM. Functionally specialized junctions between endothelial cells of lymphatic vessels. J Exp Med. 2007; 204:2349–2362. PMID: 17846148.
Article40. Yao LC, Baluk P, Srinivasan RS, Oliver G, McDonald DM. Plasticity of button-like junctions in the endothelium of airway lymphatics in development and inflammation. Am J Pathol. 2012; 180:2561–2575. PMID: 22538088.
Article41. Baluk P, Tammela T, Ator E, Lyubynska N, Achen MG, Hicklin DJ, Jeltsch M, Petrova TV, Pytowski B, Stacker SA, Yla-Herttuala S, Jackson DG, Alitalo K, McDonald DM. Pathogenesis of persistent lymphatic vessel hyperplasia in chronic airway inflammation. J Clin Invest. 2005; 115:247–257. PMID: 15668734.
Article42. Casley-Smith JR, Clodius L, Foldi M. Experimental blood vascular and lymphatic occlusion in the rabbit ear and the effect of benzopyrones. Arzneimittelforschung. 1977; 27:379–382. PMID: 577157.