Ann Dermatol.
2016 Dec;28(6):796-797. 10.5021/ad.2016.28.6.796.
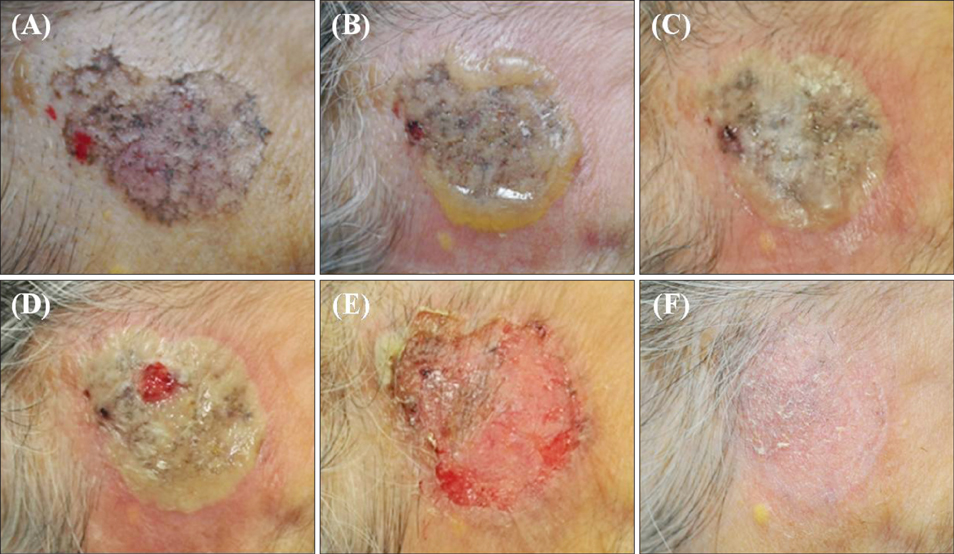
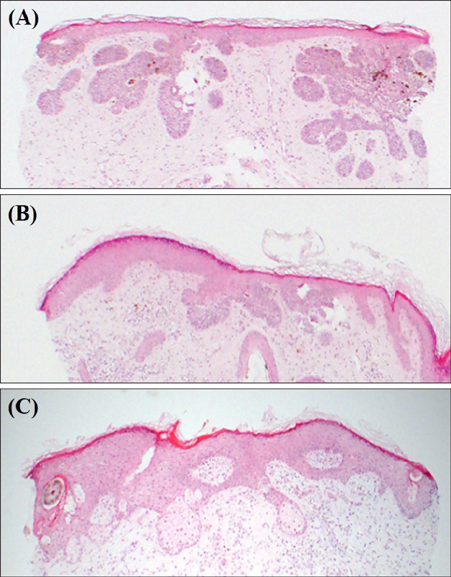
Superficial Basal Cell Carcinoma Treated with Two Cycles of Ingenol Mebutate Gel 0.015%
- Affiliations
-
- 1Department of Dermatology, St. Vincent's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea. gyongmoonkim@catholic.ac.kr
- KMID: 2368144
- DOI: http://doi.org/10.5021/ad.2016.28.6.796
Abstract
- No abstract available.
MeSH Terms
Figure
Reference
-
1. Raasch BA, Buettner PG, Garbe C. Basal cell carcinoma: histological classification and body-site distribution. Br J Dermatol. 2006; 155:401–407.
Article2. Chen CC, Chen CL. Clinical and histopathologic findings of superficial basal cell carcinoma: a comparison with other basal cell carcinoma subtypes. J Chin Med Assoc. 2006; 69:364–371.
Article3. Siller G, Rosen R, Freeman M, Welburn P, Katsamas J, Ogbourne SM. PEP005 (ingenol mebutate) gel for the topical treatment of superficial basal cell carcinoma: results of a randomized phase IIa trial. Australas J Dermatol. 2010; 51:99–105.
Article4. Cantisani C, Paolino G, Cantoresi F, Faina V, Richetta AG, Calvieri S. Superficial basal cell carcinoma successfully treated with ingenol mebutate gel 0.05%. Dermatol Ther. 2014; 27:352–354.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Development of Squamous Cell Carcinoma from Actinic Keratosis after Ingenol Mebutate Gel Use
- A Case of Molluscum Contagiosum Treated by Ingenol Mebutate (Picato®)
- In Situ Ingenol Mebutate Treatment for Squamous Cell Carcinoma of the Scalp
- Successful Treatment of Bowenoid Papulosis with Fractional CO2 Laser and Ingenol Mebutate Gel
- The Effectiveness and Local Skin Reactions of Ingenol Mebutate Gel in Patients with Actinic Keratosis