Cancer Res Treat.
2017 Jan;49(1):255-262. 10.4143/crt.2015.452.
Irinotecan Monotherapy Versus Irinotecan-Based Combination as Second-Line Chemotherapy in Advanced Gastric Cancer: A Meta-Analysis
- Affiliations
-
- 1Department of Internal Medicine, Konkuk University School of Medicine, Seoul, Korea.
- 2Department of Obstetrics and Gynecology, Konkuk University School of Medicine, Seoul, Korea. snkim@kuh.ac.kr
- KMID: 2367523
- DOI: http://doi.org/10.4143/crt.2015.452
Abstract
- PURPOSE
A meta-analysis was conducted to examine the question of whether combination regimens are more effective than monotherapy as a second-line chemotherapy in advanced gastric cancer.
MATERIALS AND METHODS
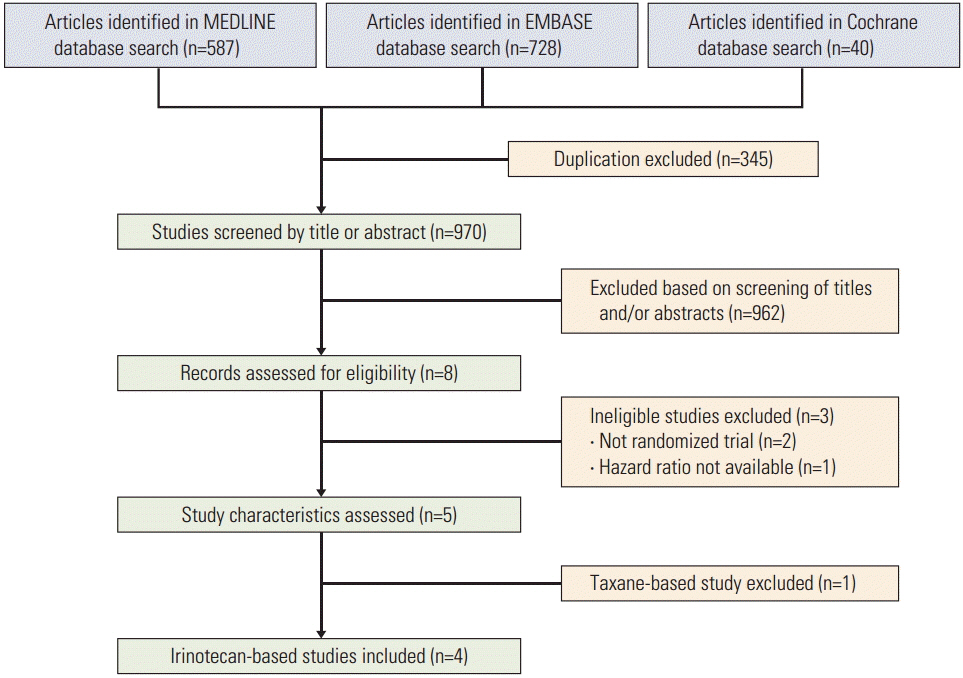
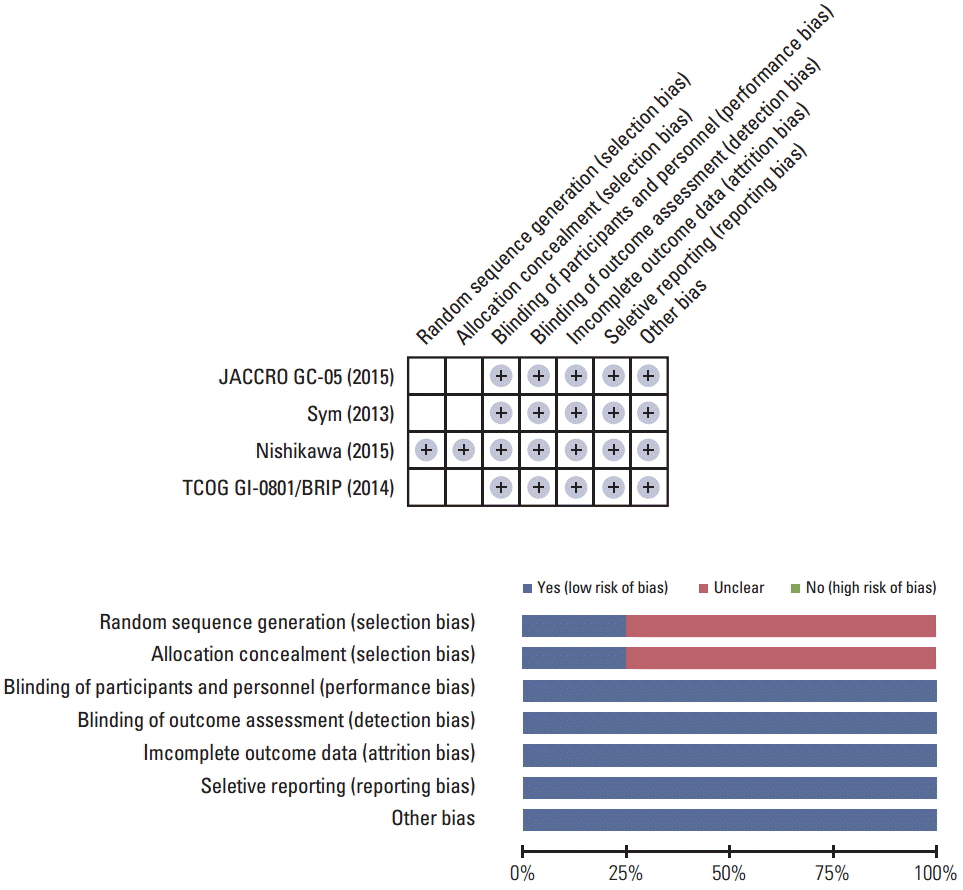
The MEDLINE and the EMBASE databases and the Cochrane Central Register for Controlled Trials were searched using appropriate keywords. Only randomized controlled trials were eligible.
RESULTS
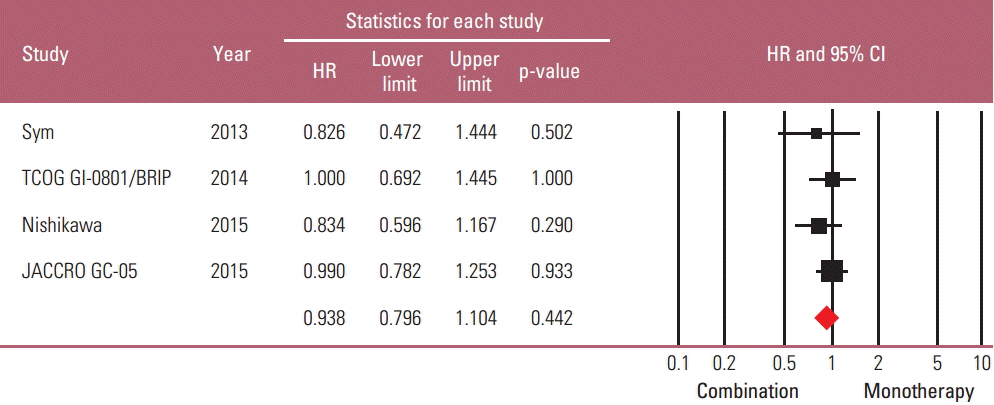
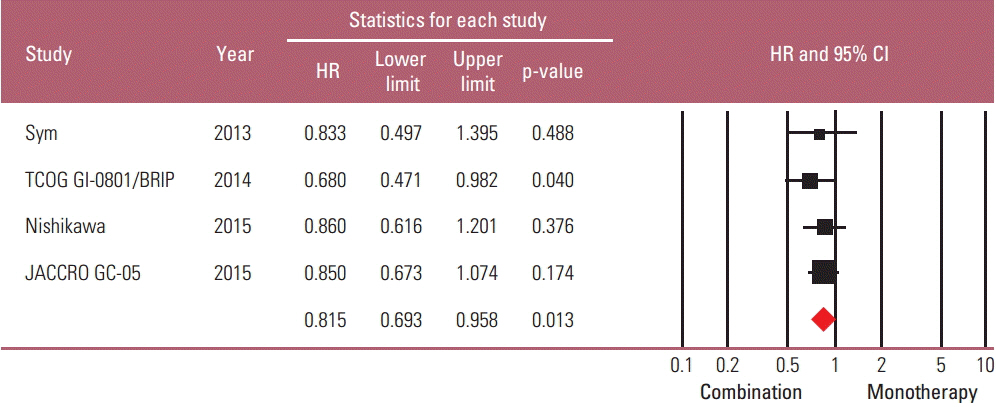
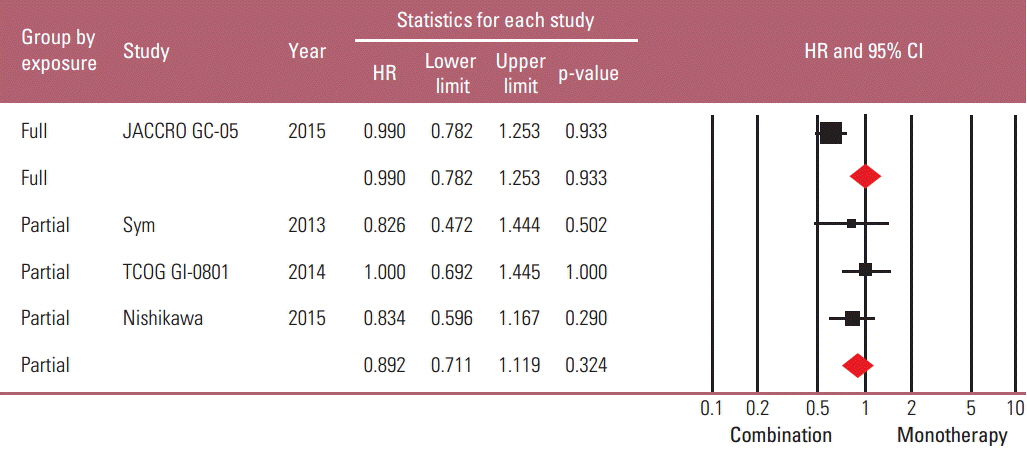
Taxane-based study is rare; thus, four irinotecan-based studies were finally included in the meta-analysis. Out of 661 patients, 331 patients were assigned to combination therapy and 330 to monotherapy. Cisplatin or fluoropyrimidine (S-1 or 5-fluorouracil) was used as a combination partner to irinotecan. The pooled hazard ratio (HR) for overall survival (OS) and for progression-free survival (PFS) was 0.938 (95% confidence interval [CI], 0.796 to 1.104; p=0.442) and 0.815 (95% CI, 0.693 to 0.958; p=0.013). In subgroup analysis according to previous exposure to a partner agent, the PFS benefit of combination was observed only in the partially exposed group (HR, 0.784; 95% CI, 0.628 to 0.980; p=0.032).
CONCLUSION
Second-line irinotecan-based combination was not associated with increased OS, but with PFS benefit, which seemed particularly significant for patients receiving combination with a new agent.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Jung KW, Won YJ, Kong HJ, Oh CM, Seo HG, Lee JS. Cancer statistics in Korea: incidence, mortality, survival and prevalence in 2010. Cancer Res Treat. 2013; 45:1–14.
Article2. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010; 127:2893–917.
Article3. Wagner AD, Unverzagt S, Grothe W, Kleber G, Grothey A, Haerting J, et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev. 2010; (3):CD004064.
Article4. Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010; 376:687–97.
Article5. Kim HS, Kim HJ, Kim SY, Kim TY, Lee KW, Baek SK, et al. Second-line chemotherapy versus supportive cancer treatment in advanced gastric cancer: a meta-analysis. Ann Oncol. 2013; 24:2850–4.
Article6. Hironaka S, Ueda S, Yasui H, Nishina T, Tsuda M, Tsumura T, et al. Randomized, open-label, phase III study comparing irinotecan with paclitaxel in patients with advanced gastric cancer without severe peritoneal metastasis after failure of prior combination chemotherapy using fluoropyrimidine plus platinum: WJOG 4007 trial. J Clin Oncol. 2013; 31:4438–44.
Article7. Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014; 15:1224–35.
Article8. Ji SH, Lim DH, Yi SY, Kim HS, Jun HJ, Kim KH, et al. A retrospective analysis of second-line chemotherapy in patients with advanced gastric cancer. BMC Cancer. 2009; 9:110.
Article9. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000; 283:2008–12.10. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009; 6:e1000097.
Article11. Higgins JP, Green S. Cochrane handbook for systematic review of interventions [Internet]. London: The Cochrane Collaboration;2011. [cited 2016 Apr 1]. Available from: http://handbook.cochrane.org/chapter_8/8_assessing_risk_of_bias_in_included_studies.htm.12. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002; 21:1539–58.
Article13. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003; 327:557–60.
Article14. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994; 50:1088–101.
Article15. Sterne JA, Egger M, Smith GD. Systematic reviews in health care: investigating and dealing with publication and other biases in meta-analysis. BMJ. 2001; 323:101–5.
Article16. Sym SJ, Hong J, Park J, Cho EK, Lee JH, Park YH, et al. A randomized phase II study of biweekly irinotecan monotherapy or a combination of irinotecan plus 5-fluorouracil/leucovorin (mFOLFIRI) in patients with metastatic gastric adenocarcinoma refractory to or progressive after first-line chemotherapy. Cancer Chemother Pharmacol. 2013; 71:481–8.
Article17. Higuchi K, Tanabe S, Shimada K, Hosaka H, Sasaki E, Nakayama N, et al. Biweekly irinotecan plus cisplatin versus irinotecan alone as second-line treatment for advanced gastric cancer: a randomised phase III trial (TCOG GI-0801/BIRIP trial). Eur J Cancer. 2014; 50:1437–45.
Article18. Nishikawa K, Fujitani K, Inagaki H, Akamaru Y, Tokunaga S, Takagi M, et al. Randomised phase III trial of second-line irinotecan plus cisplatin versus irinotecan alone in patients with advanced gastric cancer refractory to S-1 monotherapy: TRICS trial. Eur J Cancer. 2015; 51:808–16.
Article19. Tanabe K, Fujii M, Nishikawa K, Kunisaki C, Tsuji A, Matsuhashi N, et al. Phase II/III study of second-line chemotherapy comparing irinotecan-alone with S-1 plus irinotecan in advanced gastric cancer refractory to first-line treatment with S-1 (JACCRO GC-05). Ann Oncol. 2015; 26:1916–22.
Article20. Booth CM, Eisenhauer EA. Progression-free survival: meaningful or simply measurable? J Clin Oncol. 2012; 30:1030–3.
Article21. Kim JY, Ryoo HM, Bae SH, Kang BW, Chae YS, Yoon S, et al. Multi-center randomized phase II study of weekly docetaxel versus weekly docetaxel-plus-oxaliplatin as a second-line chemotherapy for patients with advanced gastric cancer. Anticancer Res. 2015; 35:3531–6.22. Kim DY, Kim JH, Lee SH, Kim TY, Heo DS, Bang YJ, et al. Phase II study of oxaliplatin, 5-fluorouracil and leucovorin in previously platinum-treated patients with advanced gastric cancer. Ann Oncol. 2003; 14:383–7.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Oxaliplatin and Leucovorin Plus Fluorouracil Versus Irinotecan and Leucovorin Plus Fluorouracil Combination Chemotherapy as a First-line Treatment in Patients with Metastatic or Recurred Gastric Adenocarcinoma
- A Case of Gastric Cancer Presenting Acute Disseminated Intravascular Coagulation Palliated with Combination Chemotherapy of Irinotecan and Cisplatin
- Irinotecan (CPT-11)-induced hemorrhagic colitis
- A Retrospective Study of First-Line Combination Chemotherapy in Advanced Colorectal Cancer: A Korean Single-Center Experience
- Syndrome of inappropriate antidiuretic hormone secretion following irinotecan-cisplatin administration as a treatment for recurrent ovarian clear cell carcinoma