Cancer Res Treat.
2017 Jan;49(1):141-149. 10.4143/crt.2016.133.
Reactive Oxygen Species Modulator 1 (Romo1) Predicts Poor Outcomes in Advanced Non-small Cell Lung Cancer Patients Treated with Platinum-Based Chemotherapy
- Affiliations
-
- 1Department of Internal Medicine, Kyung Hee University School of Medicine, Seoul, Korea.
- 2Division of Respiratory and Critical Care Medicine, Department of Internal Medicine, Korea University College of Medicine, Seoul, Korea. khin@kumc.or.kr
- 3Clinical Research Center, Asan Medical Center, Seoul, Korea.
- 4Department of Pathology, Korea University College of Medicine, Seoul, Korea.
- KMID: 2367511
- DOI: http://doi.org/10.4143/crt.2016.133
Abstract
- PURPOSE
Reactive oxygen species modulator 1 (Romo1) is a key mediator of intracellular reactive oxygen species production. However, examination of the clinical usefulness of Romo1 in cancers has been limited. We evaluated the association of Romo1 expression with clinical outcomes in advanced non-small cell lung cancer (NSCLC) patients treated with platinum-based chemotherapy.
MATERIALS AND METHODS
Romo1 expression in tumor tissue was examined by immunohistochemistry and evaluated by histological score. Survival analyses were performed according to Romo1 expression and the association between Romo1 expression and clinical parameters was evaluated.
RESULTS
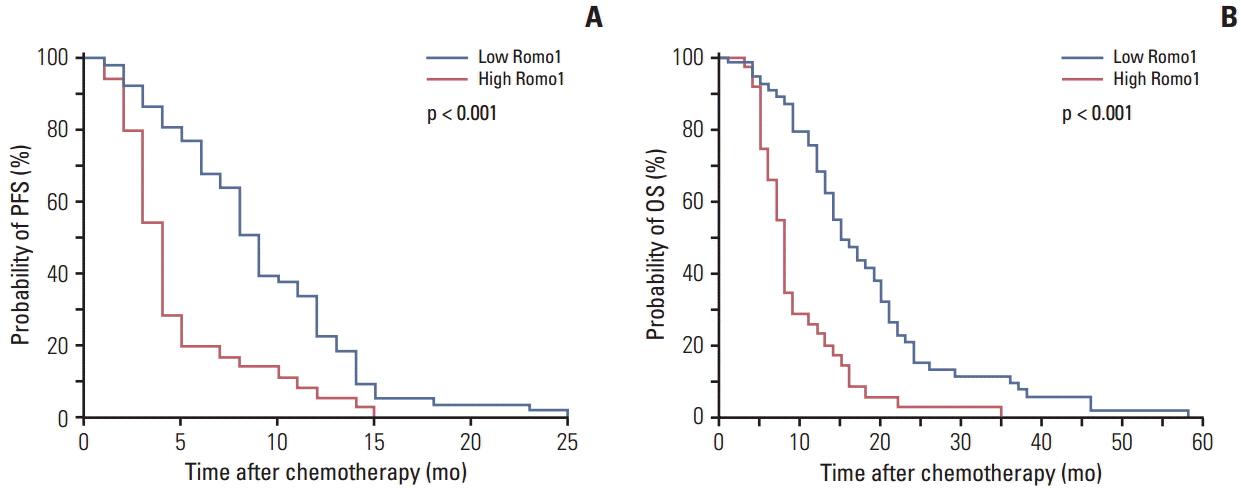
A total of 88 tumor specimens were analyzed. Significantly shorter median progression-free survival (PFS) was observed in the high Romo1 group compared with the low Romo1 group (4.5 months vs. 9.8 months, p < 0.001), and the median overall survival (OS) of the high Romo1 group was also significantly shorter than that of the low Romo1 group (8.4 months vs. 15.5 months, p < 0.001). Results of multivariate analyses showed significant association of high Romo1 expression with both poor PFS (hazard ratio [HR], 2.75; 95% confidence interval [CI], 1.71 to 4.44) and poor OS (HR, 3.99; 95% CI, 2.36 to 6.74). Results of the subgroup analysis showed a similar association regardless of tumor histology. Romo1 expression showed no association with any clinical parameter including age, sex, smoking status, stage, differentiation, or tumor histology.
CONCLUSION
Romo1 overexpression was associated with poor response to treatment and shorter survival in advanced NSCLC patients treated with platinum-based chemotherapy. Romo1 could be a potential adverse predictive marker in this setting.
MeSH Terms
Figure
Cited by 1 articles
-
Reactive Oxygen Species Modulator 1 (ROMO1), a New Potential Target for Cancer Diagnosis and Treatment
Mohammad Amin Amini, Seyed Saman Talebi, Jamshid Karimi
Chonnam Med J. 2019;55(3):136-143. doi: 10.4068/cmj.2019.55.3.136.
Reference
-
References
1. Jemal A. Global burden of cancer: opportunities for prevention. Lancet. 2012; 380:1797–9.
Article2. DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014; 64:252–71.
Article3. Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002; 346:92–8.
Article4. Slebos RJ, Kibbelaar RE, Dalesio O, Kooistra A, Stam J, Meijer CJ, et al. K-ras oncogene activation as a prognostic marker in adenocarcinoma of the lung. N Engl J Med. 1990; 323:561–5.5. Tsao MS, Aviel-Ronen S, Ding K, Lau D, Liu N, Sakurada A, et al. Prognostic and predictive importance of p53 and RAS for adjuvant chemotherapy in non small-cell lung cancer. J Clin Oncol. 2007; 25:5240–7.6. Miller VA, Riely GJ, Zakowski MF, Li AR, Patel JD, Heelan RT, et al. Molecular characteristics of bronchioloalveolar carcinoma and adenocarcinoma, bronchioloalveolar carcinoma subtype, predict response to erlotinib. J Clin Oncol. 2008; 26:1472–8.
Article7. Roberts PJ, Stinchcombe TE. KRAS mutation: should we test for it, and does it matter? J Clin Oncol. 2013; 31:1112–21.
Article8. Altaha R, Liang X, Yu JJ, Reed E. Excision repair cross complementing-group 1: gene expression and platinum resistance. Int J Mol Med. 2004; 14:959–70.9. Rosell R, Lord RV, Taron M, Reguart N. DNA repair and cisplatin resistance in non-small-cell lung cancer. Lung Cancer. 2002; 38:217–27.
Article10. Lord RV, Brabender J, Gandara D, Alberola V, Camps C, Domine M, et al. Low ERCC1 expression correlates with prolonged survival after cisplatin plus gemcitabine chemotherapy in non-small cell lung cancer. Clin Cancer Res. 2002; 8:2286–91.11. Olaussen KA, Dunant A, Fouret P, Brambilla E, Andre F, Haddad V, et al. DNA repair by ERCC1 in non-small-cell lung cancer and cisplatin-based adjuvant chemotherapy. N Engl J Med. 2006; 355:983–91.
Article12. Simon GR, Sharma S, Cantor A, Smith P, Bepler G. ERCC1 expression is a predictor of survival in resected patients with non-small cell lung cancer. Chest. 2005; 127:978–83.
Article13. Cobo M, Isla D, Massuti B, Montes A, Sanchez JM, Provencio M, et al. Customizing cisplatin based on quantitative excision repair cross-complementing 1 mRNA expression: a phase III trial in non-small-cell lung cancer. J Clin Oncol. 2007; 25:2747–54.
Article14. Chung YM, Kim JS, Yoo YD. A novel protein, Romo1, induces ROS production in the mitochondria. Biochem Biophys Res Commun. 2006; 347:649–55.
Article15. Na AR, Chung YM, Lee SB, Park SH, Lee MS, Yoo YD. A critical role for Romo1-derived ROS in cell proliferation. Biochem Biophys Res Commun. 2008; 369:672–8.
Article16. Hwang IT, Chung YM, Kim JJ, Chung JS, Kim BS, Kim HJ, et al. Drug resistance to 5-FU linked to reactive oxygen species modulator 1. Biochem Biophys Res Commun. 2007; 359:304–10.
Article17. Lee SH, Lee JS, Lee EJ, Min KH, Hur GY, Lee SH, et al. Serum reactive oxygen species modulator 1 (Romo1) as a potential diagnostic biomarker for non-small cell lung cancer. Lung Cancer. 2014; 85:175–81.
Article18. Lee SH, Min JW, Lee JS, Kim CH, Yoo YD, Lee EJ, et al. Reactive oxygen species modulator 1 (Romo1) overexpression is an independent predictor of poor survival in NSCLC patients who undergo surgical resection. Lung Cancer. 2015; 87:45–52.
Article19. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45:228–47.
Article20. Chung YM, Lee SB, Kim HJ, Park SH, Kim JJ, Chung JS, et al. Replicative senescence induced by Romo1-derived reactive oxygen species. J Biol Chem. 2008; 283:33763–71.
Article21. Chung JS, Park S, Park SH, Park ER, Cha PH, Kim BY, et al. Overexpression of Romo1 promotes production of reactive oxygen species and invasiveness of hepatic tumor cells. Gastroenterology. 2012; 143:1084–94.
Article22. Sosa V, Moline T, Somoza R, Paciucci R, Kondoh H, LLeonart ME. Oxidative stress and cancer: an overview. Ageing Res Rev. 2013; 12:376–90.
Article23. Pelicano H, Carney D, Huang P. ROS stress in cancer cells and therapeutic implications. Drug Resist Updat. 2004; 7:97–110.
Article24. Chung JS, Lee SB, Park SH, Kang ST, Na AR, Chang TS, et al. Mitochondrial reactive oxygen species originating from Romo1 exert an important role in normal cell cycle progression by regulating p27(Kip1) expression. Free Radic Res. 2009; 43:729–37.25. Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009; 361:947–57.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Association of COVID-19 and Reactive Oxygen Species Modulator 1 (ROMO1) with Oxidative Stress
- The Association of Oxidative Stress and Reactive Oxygen Species Modulator 1 (ROMO1) with Infertility: A Mini Review
- Reactive Oxygen Species Modulator 1 (ROMO1), a New Potential Target for Cancer Diagnosis and Treatment
- Mitochondrial Reactive Oxygen Species Production Mediated by Romo1 Expression
- Influence of Methylenetetrahydrofolate Reductase C677T Polymorphism on the Risk of Lung Cancer and the Clinical Response to Platinum-Based Chemotherapy for Advanced Non-Small Cell Lung Cancer: An Updated Meta-Analysis