Clin Exp Vaccine Res.
2017 Jan;6(1):38-44. 10.7774/cevr.2017.6.1.38.
Comparison of immunogenicity and safety of an influenza vaccine administered concomitantly with a 13-valent pneumococcal conjugate vaccine or 23-valent polysaccharide pneumococcal vaccine in the elderly
- Affiliations
-
- 1Division of Infectious Diseases, Department of Internal Medicine, Hallym University College of Medicine, Chuncheon, Korea.
- 2Division of Infectious Diseases, Department of Internal Medicine, Korea University College of Medicine, Seoul, Korea. heejinmd@korea.ac.kr
- 3Asian Pacific Influenza Institute (APII), Seoul, Korea.
- KMID: 2366890
- DOI: http://doi.org/10.7774/cevr.2017.6.1.38
Abstract
- PURPOSE
Previous studies have demonstrated the immunogenicity and safety of the co-administration of the trivalent inactivated influenza vaccine (IIV3) with the polysaccharide pneumococcal vaccine (PPV) or pneumococcal conjugate vaccine (PCV). However, there is no direct comparison study that evaluates the immunogenicity and safety of IIV3 given concomitantly with PCV13 or PPV23 in the elderly.
MATERIALS AND METHODS
During the 2012-2013 influenza vaccination period, 224 healthy elderly volunteers aged 65 years and older randomly received IIV3 given concomitantly with either PCV13 (PCV13+IIV3) or PPV23 (PPV23+IIV3) in a 1:1 ratio. Serum hemagglutination-inhibiting antibodies for IIV3 were measured at the time of vaccination and 1 month after vaccination. Adverse events were recorded prospectively in a clinical diary during a 7-day period.
RESULTS
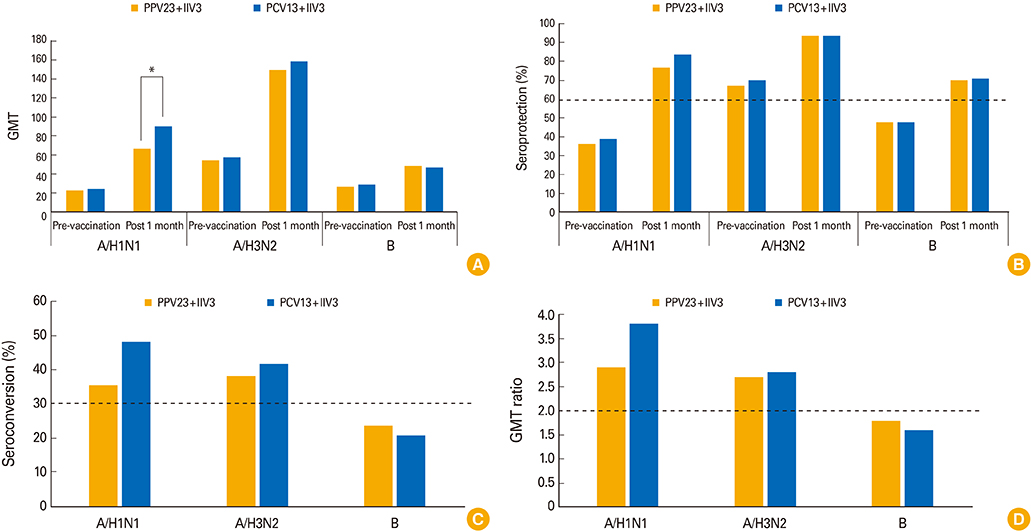
A total of 220 participants blood samples for analysis of immunogenicity and kept a clinical diary for safety analysis (PCV13+IIV3, n=110; PPV23+IIV3, n=110). One month after vaccination, both groups satisfied the Committee for Medical Products for Human Use criteria for A/H1N1, A/H3N2 and B strains, showing comparable seroprotection rates, seroconversion rates and geometric mean titer fold. The assessments of immunogenicity were similar in both groups. The most common local and systemic reactions were pain at the injection site and generalized myalgia. They were generally mild or moderate in intensity. The adverse events were not statistically different between the two groups.
CONCLUSION
PCV13+IIV3 and PPV23+IIV3 demonstrated similar immunogenicity and safety in the elderly.
Keyword
MeSH Terms
Figure
Reference
-
1. Walter ND, Taylor TH, Shay DK, et al. Influenza circulation and the burden of invasive pneumococcal pneumonia during a non-pandemic period in the United States. Clin Infect Dis. 2010; 50:175–183.
Article2. Grabowska K, Hogberg L, Penttinen P, Svensson A, Ekdahl K. Occurrence of invasive pneumococcal disease and number of excess cases due to influenza. BMC Infect Dis. 2006; 6:58.
Article3. Mangtani P, Cumberland P, Hodgson CR, Roberts JA, Cutts FT, Hall AJ. A cohort study of the effectiveness of influenza vaccine in older people, performed using the United Kingdom general practice research database. J Infect Dis. 2004; 190:1–10.
Article4. Nichol KL, Nordin JD, Nelson DB, Mullooly JP, Hak E. Effectiveness of influenza vaccine in the community-dwelling elderly. N Engl J Med. 2007; 357:1373–1381.
Article5. Mooney JD, Weir A, McMenamin J, et al. The impact and effectiveness of pneumococcal vaccination in Scotland for those aged 65 and over during winter 2003/2004. BMC Infect Dis. 2008; 8:53.
Article6. Vila-Corcoles A, Ochoa-Gondar O, Guzman JA, et al. Effectiveness of the 23-valent polysaccharide pneumococcal vaccine against invasive pneumococcal disease in people 60 years or older. BMC Infect Dis. 2010; 10:73.
Article7. Nichol KL. The additive benefits of influenza and pneumococcal vaccinations during influenza seasons among elderly persons with chronic lung disease. Vaccine. 1999; 17:Suppl 1. S91–S93.
Article8. Furumoto A, Ohkusa Y, Chen M, et al. Additive effect of pneumococcal vaccine and influenza vaccine on acute exacerbation in patients with chronic lung disease. Vaccine. 2008; 26:4284–4289.
Article9. Christenson B, Hedlund J, Lundbergh P, Ortqvist A. Additive preventive effect of influenza and pneumococcal vaccines in elderly persons. Eur Respir J. 2004; 23:363–368.
Article10. Fedson DS, Nicolas-Spony L, Klemets P, et al. Pneumococcal polysaccharide vaccination for adults: new perspectives for Europe. Expert Rev Vaccines. 2011; 10:1143–1167.
Article11. Song JY, Cheong HJ, Heo JY, et al. Outpatient-based pneumococcal vaccine campaign and survey of perceptions about pneumococcal vaccination in patients and doctors. Yonsei Med J. 2013; 54:469–475.
Article12. Honkanen PO, Keistinen T, Kivela SL. Reactions following administration of influenza vaccine alone or with pneumococcal vaccine to the elderly. Arch Intern Med. 1996; 156:205–208.
Article13. Schwarz TF, Flamaing J, Rumke HC, et al. A randomized, double-blind trial to evaluate immunogenicity and safety of 13-valent pneumococcal conjugate vaccine given concomitantly with trivalent influenza vaccine in adults aged ≥ 65 years. Vaccine. 2011; 29:5195–5202.
Article14. Hannoun C, Megas F, Piercy J. Immunogenicity and protective efficacy of influenza vaccination. Virus Res. 2004; 103:133–138.
Article15. Song JY, Cheong HJ, Woo HJ, et al. Immunogenicity and safety of trivalent inactivated influenza vaccine: a randomized, double-blind, multi-center, phase 3 clinical trial in a vaccine-limited country. J Korean Med Sci. 2011; 26:191–195.
Article16. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Biologics Evaluation and Research. Guidance for industry toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials. Rockville: U.S: Department of Health and Human Services;2007.17. Fletcher TJ, Tunnicliffe WS, Hammond K, Roberts K, Ayres JG. Simultaneous immunisation with influenza vaccine and pneumococcal polysaccharide vaccine in patients with chronic respiratory disease. BMJ. 1997; 314:1663–1665.
Article18. Frenck RW Jr, Gurtman A, Rubino J, et al. Randomized, controlled trial of a 13-valent pneumococcal conjugate vaccine administered concomitantly with an influenza vaccine in healthy adults. Clin Vaccine Immunol. 2012; 19:1296–1303.
Article19. Song JY, Cheong HJ, Tsai TF, et al. Immunogenicity and safety of concomitant MF59-adjuvanted influenza vaccine and 23-valent pneumococcal polysaccharide vaccine administration in older adults. Vaccine. 2015; 33:4647–4652.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Efficacy and effectiveness of extended-valency pneumococcal conjugate vaccines
- Use of vaccine in the era of antimicrobial resistance: need of effective pneumococcal vaccines
- Pneumococcal vaccine
- Immunogenicity of 7-valent pneumococcal conjugate vaccine related to booster immunization in Korean children
- Efficacy of 23-valent Pneumococcal Polysaccharide Vaccine in Steroid Responsive Nephrotic Syndrome