J Nutr Health.
2016 Dec;49(6):420-428. 10.4163/jnh.2016.49.6.420.
Protective effects of Cirsium setidens ethanolic extracts against alcoholic fatty liver injury in rats
- Affiliations
-
- 1Department of Food and Nutrition, Kyung Hee University, Seoul 02447, Korea. jchung@khu.ac.kr
- KMID: 2366713
- DOI: http://doi.org/10.4163/jnh.2016.49.6.420
Abstract
- PURPOSE
In this study, we investigated the effects of Cirsium setidens ethanolic extract (CS) on the development of alcoholic fatty liver and associated injury.
METHODS
Sprague-Dawley male rats were fed either Lieber-DeCarli control (C) or ethanol (35.5% of total calories) liquid diet with 0 (E), 100 mg/kgBW CS (E+LCS), or 500 mg/kgBW CS (E+HCS) for 8 weeks. Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities as well as TG and cholesterol concentrations in the serum and liver tissues were measured by colorimetric assays. Liver histopathology was examined by Hematoxylin-eosin staining of the fixed liver tissues. Protein levels of phosphorylated-AMP activated protein kinase (p-AMPK), phosphorylated-acetyl CoA carboxylase (p-ACC), phosphorylated-nuclear factor kappa B (p-NFκB), and TNFα were measured by Western blot analyses.
RESULTS
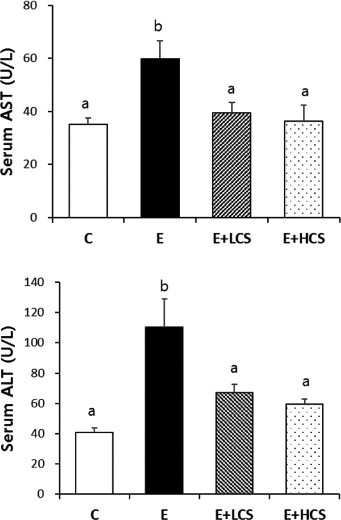
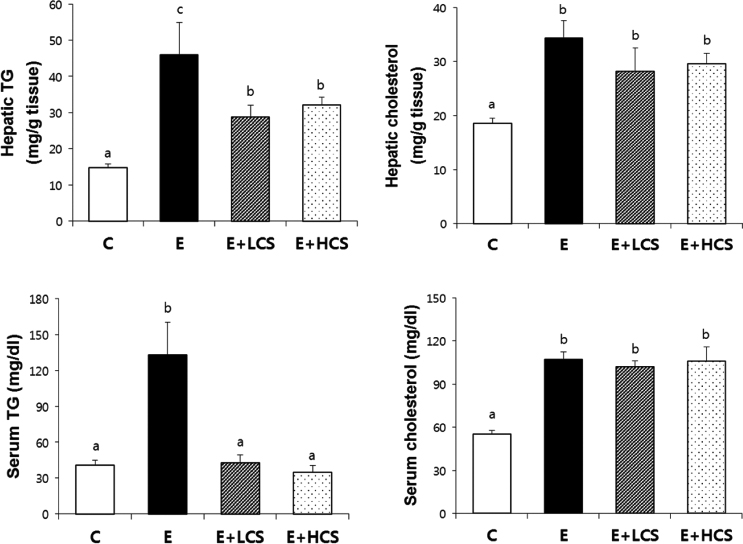
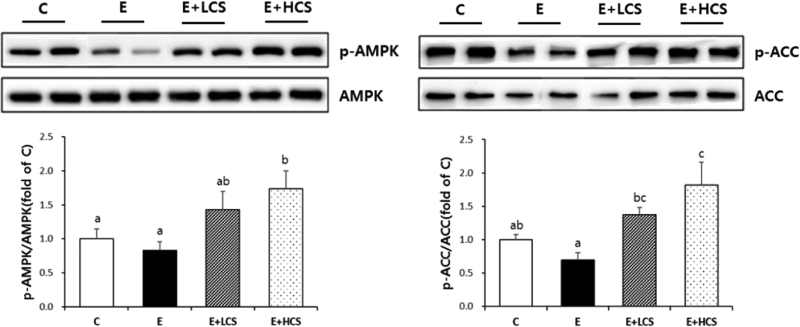
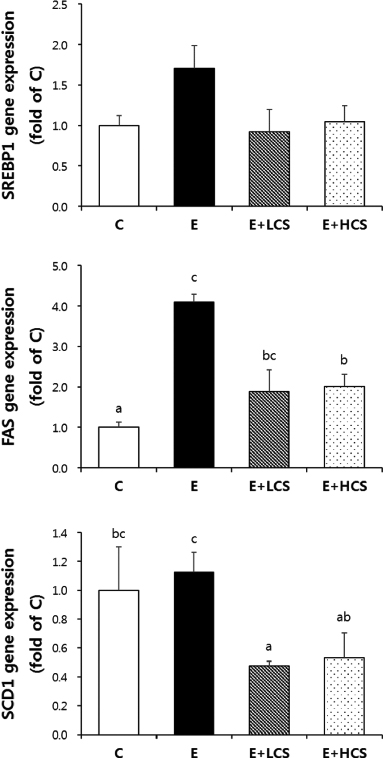
Both doses of CS markedly suppressed alcohol-induced lipid droplets accumulation in the liver tissues and significantly inhibited alcohol-induced increases in activities of serum ALT and serum AST. Similarly, CS significantly reduced hepatic and serum TG concentrations. Compared to groups fed alcohol only, CS supplementation strongly increased hepatic levels of p-AMPK and p-ACC. Further, CS significantly inhibited alcohol-induced phosphorylation of NFκB, which was associated with reduced hepatic protein levels of TNFα.
CONCLUSION
Our data demonstrated that CS has a protective effect against alcoholic liver injury, which was associated with activation of AMPK and inhibition of NFκB.
Keyword
MeSH Terms
-
Alanine Transaminase
Alcoholics*
AMP-Activated Protein Kinases
Animals
Aspartate Aminotransferases
Blotting, Western
Cholesterol
Cirsium*
Diet
Ethanol*
Fatty Liver, Alcoholic*
Humans
Lipid Droplets
Liver
Liver Diseases, Alcoholic
Male
NF-kappa B
Phosphorylation
Protein Kinases
Rats*
Rats, Sprague-Dawley
AMP-Activated Protein Kinases
Alanine Transaminase
Aspartate Aminotransferases
Cholesterol
Ethanol
NF-kappa B
Protein Kinases
Figure
Reference
-
1. Ministry of Health and Welfare. Korea Centers for Disease Control and Prevention. Korea Health Statistics 2013: Korea National Health and Nutrition Examination Survey (KNHANES VI-1). Cheongju: Korea Centers for Disease Control and Prevention;2014.2. Lee SM, Yoon DY, Paik JH, Hyun KR, Kang HR. Socioeconomic impacts of major health risk factor and effectiveness evaluation of regulatory policy report. Seoul: National Health Insurance Service;2015.3. Purohit V, Gao B, Song BJ. Molecular mechanisms of alcoholic fatty liver. Alcohol Clin Exp Res. 2009; 33(2):191–205.
Article4. Deleuran T, Grønbaek H, Vilstrup H, Jepsen P. Cirrhosis and mortality risks of biopsy-verified alcoholic pure steatosis and steatohepatitis: a nationwide registry-based study. Aliment Pharmacol Ther. 2012; 35(11):1336–1342.
Article5. Haflidadottir S, Jonasson JG, Norland H, Einarsdottir SO, Kleiner DE, Lund SH, Björnsson ES. Long-term follow-up and liver-related death rate in patients with non-alcoholic and alcoholic related fatty liver disease. BMC Gastroenterol. 2014; 14(1):166.
Article6. Lee WB, Kwon HC, Cho OR, Lee KC, Choi SU, Baek NI, Lee KR. Phytochemical constituents of Cirsium setidens Nakai and their cytotoxicity against human cancer cell lines. Arch Pharm Res. 2002; 25(5):628–635.7. Lee SH, Jin YS, Heo SI, Shim TH, Sa JH, Choi DS, Wang MH. Composition analysis and antioxidative activity from different organs of Cirsium setidens Nakai. Korean J Food Sci Technol. 2006; 38(4):571–576.8. Thao NT, Cuong TD, Hung TM, Lee JH, Na M, Son JK, Jung HJ, Fang Z, Woo MH, Choi JS, Min BS. Simultaneous determination of bioactive flavonoids in some selected Korean thistles by high-performance liquid chromatography. Arch Pharm Res. 2011; 34(3):455–461.
Article9. Jeong DM, Jung HA, Choi JS. Comparative antioxidant activity and HPLC profiles of some selected Korean thistles. Arch Pharm Res. 2008; 31(1):28–33.
Article10. Lee JH, Jung HK, Han YS, Yoon YM, Yun CW, Sun HY, Cho HW, Lee SH. Antioxidant effects of Cirsium setidens extract on oxidative stress in human mesenchymal stem cells. Mol Med Rep. 2016; 14(4):3777–3784.
Article11. Chung MJ, Lee S, Park YI, Lee J, Kwon KH. Neuroprotective effects of phytosterols and flavonoids from Cirsium setidens and Aster scaber in human brain neuroblastoma SK-N-SH cells. Life Sci. 2016; 148:173–182.
Article12. Ahn MJ, Hur SJ, Kim EH, Lee SH, Shin JS, Kim MK, Uchizono JA, Whang WK, Kim DS. Scopoletin from Cirsium setidens Increases Melanin Synthesis via CREB phosphorylation in B16F10 cells. Korean J Physiol Pharmacol. 2014; 18(4):307–311.13. Yoo YM, Nam JH, Kim MY, Choi J, Park HJ. Pectolinarin and pectolinarigenin of cirsium setidens prevent the hepatic injury in rats caused by D-galactosamine via an antioxidant mechanism. Biol Pharm Bull. 2008; 31(4):760–764.
Article14. Yoo YM, Nam JH, Kim MY, Choi J, Park HJ. Pectolinarin and pectolinarigenin of Cirsium setidens prevent the hepatic injury in rats caused by D-galactosamine via an antioxidant mechanism. Biol Pharm Bull. 2008; 31(4):760–764.
Article15. Long YC, Zierath JR. AMP-activated protein kinase signaling in metabolic regulation. J Clin Invest. 2006; 116(7):1776–1783.
Article16. Towler MC, Hardie DG. AMP-activated protein kinase in metabolic control and insulin signaling. Circ Res. 2007; 100(3):328–341.
Article17. You M, Matsumoto M, Pacold CM, Cho WK, Crabb DW. The role of AMP-activated protein kinase in the action of ethanol in the liver. Gastroenterology. 2004; 127(6):1798–1808.
Article18. García-Villafranca J, Guillén A, Castro J. Ethanol consumption impairs regulation of fatty acid metabolism by decreasing the activity of AMP-activated protein kinase in rat liver. Biochimie. 2008; 90(3):460–466.
Article19. Mathews S, Xu M, Wang H, Bertola A, Gao B. Animals models of gastrointestinal and liver diseases. Animal models of alcohol-induced liver disease: pathophysiology, translational relevance, and challenges. Am J Physiol Gastrointest Liver Physiol. 2014; 306(10):G819–G823.
Article20. Steinberg GR, Kemp BE. AMPK in health and disease. Physiol Rev. 2009; 89(3):1025–1278.
Article21. Noh H, Lee H, Kim E, Mu L, Rhee YK, Cho CW, Chung J. Inhibitory effect of a Cirsium setidens extract on hepatic fat accumulation in mice fed a high-fat diet via the induction of fatty acid beta-oxidation. Biosci Biotechnol Biochem. 2013; 77(7):1424–1429.22. Viollet B, Lantier L, Devin-Leclerc J, Hebrard S, Amouyal C, Mounier R, Foretz M, Andreelli F. Targeting the AMPK pathway for the treatment of type 2 diabetes. Front Biosci (Landmark Ed). 2009; 14:3380–3400.
Article23. Steinberg GR, Kemp BE. AMPK in health and disease. Physiol Rev. 2009; 89(3):1025–1078.
Article24. Noh H, Lee H, Kim E, Mu L, Rhee YK, Cho CW, Chung J. Inhibitory effect of a Cirsium setidens extract on hepatic fat accumulation in mice fed a high-fat diet via the induction of fatty acid beta-oxidation. Biosci Biotechnol Biochem. 2013; 77(7):1424–1429.25. Lee YJ, Kim DB, Lee JS, Cho JH, Kim BK, Choi HS, Lee BY, Lee OH. Antioxidant activity and anti-adipogenic effects of wild herbs mainly cultivated in Korea. Molecules. 2013; 18(10):12937–12950.
Article26. Ding RB, Tian K, Huang LL, He CW, Jiang Y, Wang YT, Wan JB. Herbal medicines for the prevention of alcoholic liver disease: a review. J Ethnopharmacol. 2012; 144(3):457–465.
Article27. You M, Liang X, Ajmo JM, Ness GC. Involvement of mammalian sirtuin 1 in the action of ethanol in the liver. Am J Physiol Gastrointest Liver Physiol. 2008; 294(4):G892–G898.
Article28. Nagata K, Suzuki H, Sakaguchi S. Common pathogenic mechanism in development progression of liver injury caused by non-alcoholic or alcoholic steatohepatitis. J Toxicol Sci. 2007; 32(5):453–468.
Article29. McClain CJ, Hill DB, Song Z, Deaciuc I, Barve S. Monocyte activation in alcoholic liver disease. Alcohol. 2002; 27(1):53–61.
Article30. Lee SH, Heo SI, Li L, Lee MJ, Wang MH. Antioxidant and hepatoprotective activities of Cirsium setidens Nakai against CCl4-induced liver damage. Am J Chin Med. 2008; 36(1):107–114.
Article31. U.S. Department of Health & Human Services. U.S. Food & Drug Administration. Center for Drug Evaluation and Research (US). Guidance for industry: estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers. Rockville (MD): Center for Drug Evaluation and Research;2005.32. Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008; 22(3):659–661.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- An Activation of Peroxisome Proliferator-Activated Receptor delta Attenuate Alcoholic Liver Disease and Nonalcoholic Fatty Liver Disease in Rats
- Should you advocate for hepatocellular carcinomasurveillance in patients with alcohol-related liverdisease or non-alcoholic fatty liver disease?
- Silymarin's Protective Effects and Possible Mechanisms on Alcoholic Fatty Liver for Rats
- Antioxidant and antidiabetic activities of extracts from Cirsium japonicum roots
- Ultrastructural changes of hepatic stellate cells in the space of Disse in alcoholic fatty liver