J Korean Med Sci.
2017 Mar;32(3):522-527. 10.3346/jkms.2017.32.3.522.
Frequency and Clinical Characteristics of Hydroxychloroquine Retinopathy in Korean Patients with Rheumatologic Diseases
- Affiliations
-
- 1Department of Ophthalmology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. sangjinkim@skku.edu
- 2Division of Rheumatology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 2366651
- DOI: http://doi.org/10.3346/jkms.2017.32.3.522
Abstract
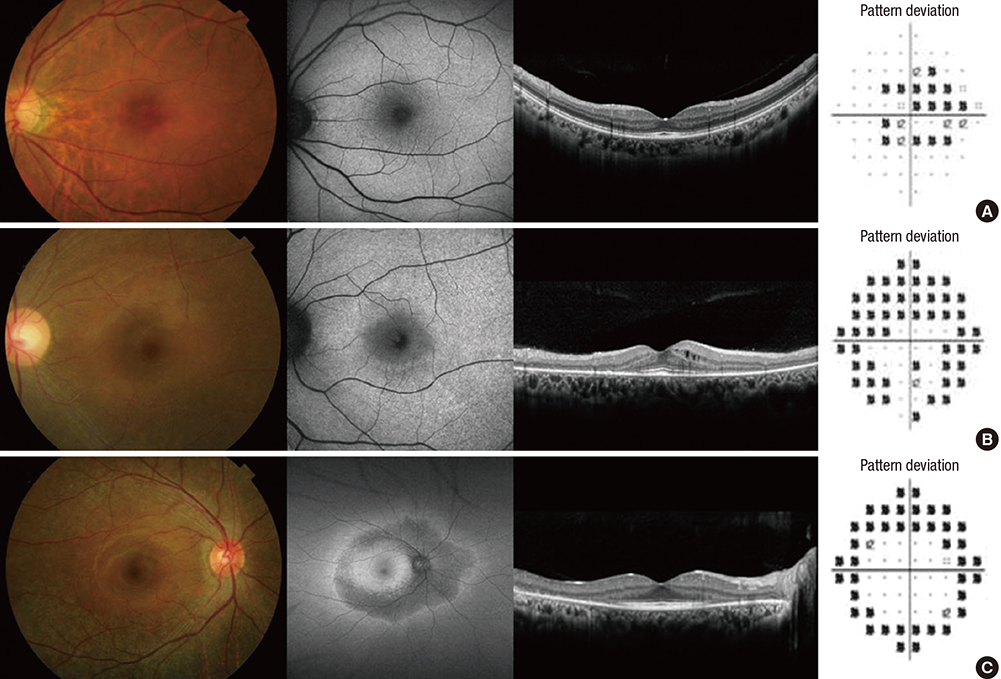
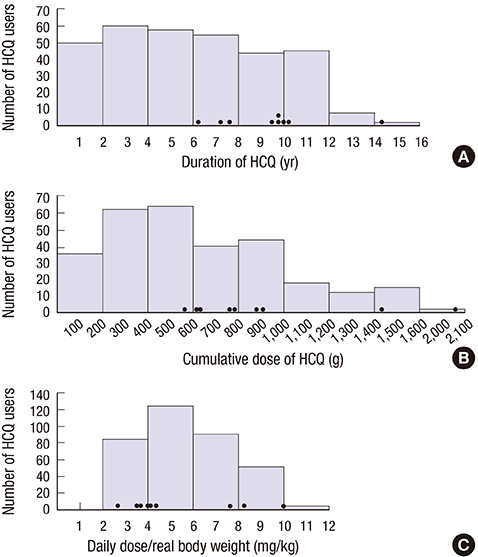
- This study aimed to evaluate the frequency and clinical characteristics of hydroxychloroquine (HCQ) retinopathy in Korean patients with rheumatologic diseases. We retrospectively reviewed medical records of 310 patients taking HCQ. Ophthalmic examinations included spectral-domain optical coherence tomography (SD-OCT), automated visual field test, and fundus autofluorescence. The severity of retinopathy was categorized as early, moderate, or severe, and the location was categorized as parafoveal, pericentral, or mixed pattern. Among 310 patients, 9 patients (2.9%) were diagnosed as HCQ retinopathy. Among the patients with HCQ use ≥ 5 years (n = 174), the frequency was 5.2%. Only 1 (11.1%) of the 9 patients was symptomatic. The mean daily dose per kilogram of real body weight of the 9 patients was 5.6 mg, and only 3 had used 6.5 mg or more. Four of the 9 patients had severe HCQ retinopathy. Six of the 9 patients showed pericentral or mixed pattern of retinal damage. Consequently, the frequency of HCQ retinopathy in Korean patients was not low, especially when administered at a high cumulative dose and for a long duration. Screening of HCQ retinopathy by the recommended guidelines that include SD-OCT seems useful and should be done to detect retinal damage earlier in patients with chronic exposure to HCQ.
MeSH Terms
Figure
Reference
-
1. Dörner T. Therapy: hydroxychloroquine in SLE: old drug, new perspectives. Nat Rev Rheumatol. 2010; 6:10–11.2. Cansu DU, Korkmaz C. Hypoglycaemia induced by hydroxychloroquine in a non-diabetic patient treated for RA. Rheumatology (Oxford). 2008; 47:378–379.3. Wolfe F, Marmor MF. Rates and predictors of hydroxychloroquine retinal toxicity in patients with rheumatoid arthritis and systemic lupus erythematosus. Arthritis Care Res (Hoboken). 2010; 62:775–784.4. Levy GD, Munz SJ, Paschal J, Cohen HB, Pince KJ, Peterson T. Incidence of hydroxychloroquine retinopathy in 1,207 patients in a large multicenter outpatient practice. Arthritis Rheum. 1997; 40:1482–1486.5. Mavrikakis I, Sfikakis PP, Mavrikakis E, Rougas K, Nikolaou A, Kostopoulos C, Mavrikakis M. The incidence of irreversible retinal toxicity in patients treated with hydroxychloroquine: a reappraisal. Ophthalmology. 2003; 110:1321–1326.6. Marmor MF, Carr RE, Easterbrook M, Farjo AA, Mieler WF; American Academy of Ophthalmology. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy: a report by the American Academy of Ophthalmology. Ophthalmology. 2002; 109:1377–1382.7. Marmor MF, Kellner U, Lai TY, Lyons JS, Mieler WF; American Academy of Ophthalmology. Revised recommendations on screening for chloroquine and hydroxychloroquine retinopathy. Ophthalmology. 2011; 118:415–422.8. Marmor MF, Kellner U, Lai TY, Melles RB, Mieler WF; American Academy of Ophthalmology. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy (2016 revision). Ophthalmology. 2016; 123:1386–1394.9. Browning DJ, Lee C. Relative sensitivity and specificity of 10-2 visual fields, multifocal electroretinography, and spectral domain optical coherence tomography in detecting hydroxychloroquine and chloroquine retinopathy. Clin Ophthalmol. 2014; 8:1389–1399.10. Asensio-Sánchez VM. SD-OCT As screening test for hydroxychloroquine retinopathy: the «flying saucer» sign. Arch Soc Esp Oftalmol. 2015; 90:338–340.11. Melles RB, Marmor MF. The risk of toxic retinopathy in patients on long-term hydroxychloroquine therapy. JAMA Ophthalmol. 2014; 132:1453–1460.12. Melles RB, Marmor MF. Pericentral retinopathy and racial differences in hydroxychloroquine toxicity. Ophthalmology. 2015; 122:110–116.13. Elder M, Rahman AM, McLay J. Early paracentral visual field loss in patients taking hydroxychloroquine. Arch Ophthalmol. 2006; 124:1729–1733.14. Michaelides M, Stover NB, Francis PJ, Weleber RG. Retinal toxicity associated with hydroxychloroquine and chloroquine: risk factors, screening, and progression despite cessation of therapy. Arch Ophthalmol. 2011; 129:30–39.15. Bergholz R, Schroeter J, Rüther K. Evaluation of risk factors for retinal damage due to chloroquine and hydroxychloroquine. Br J Ophthalmol. 2010; 94:1637–1642.16. Lai TY, Ngai JW, Chan WM, Lam DS. Visual field and multifocal electroretinography and their correlations in patients on hydroxychloroquine therapy. Doc Ophthalmol. 2006; 112:177–187.17. Marmor MF. Comparison of screening procedures in hydroxychloroquine toxicity. Arch Ophthalmol. 2012; 130:461–469.18. Lee DH, Melles RB, Joe SG, Lee JY, Kim JG, Lee CK, Yoo B, Koo BS, Kim JT, Marmor MF, et al. Pericentral hydroxychloroquine retinopathy in Korean patients. Ophthalmology. 2015; 122:1252–1256.19. Cambiaggi A. Unusual ocular lesions in a case of systemic lupus erythematosus. AMA Arch Opthalmol. 1957; 57:451–453.20. Finbloom DS, Silver K, Newsome DA, Gunkel R. Comparison of hydroxychloroquine and chloroquine use and the development of retinal toxicity. J Rheumatol. 1985; 12:692–694.21. Yam JC, Kwok AK. Ocular toxicity of hydroxychloroquine. Hong Kong Med J. 2006; 12:294–304.22. Silman A, Shipley M. Ophthalmological monitoring for hydroxychloroquine toxicity: a scientific review of available data. Br J Rheumatol. 1997; 36:599–601.23. Tehrani R, Ostrowski RA, Hariman R, Jay WM. Ocular toxicity of hydroxychloroquine. Semin Ophthalmol. 2008; 23:201–209.24. Hickley NM, Al-Maskari A, McKibbin M. Chloroquine and hydroxychloroquine toxicity. Arch Ophthalmol. 2011; 129:1506–1507.25. Mills PV, Beck M, Power BJ. Assessment of the retinal toxicity of hydroxychloroquine. Trans Ophthalmol Soc U K. 1981; 101:109–113.26. Farrell DF. Retinal toxicity to antimalarial drugs: chloroquine and hydroxychloroquine: a neurophysiologic study. Clin Ophthalmol. 2012; 6:377–383.27. Moschos MN, Moschos MM, Apostolopoulos M, Mallias JA, Bouros C, Theodossiadis GP. Assessing hydroxychloroquine toxicity by the multifocal ERG. Doc Ophthalmol. 2004; 108:47–53.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hydroxychloroquine Retinopathy
- Hydroxychloroquine Retinopathy Update
- Successful Treatment of Hydroxychloroquine-Induced Recalcitrant Acute Generalized Exanthematous Pustulosis with Cyclosporine: Case Report and Literature Review
- Prevalence and Risk Factors of Hydroxychloroquine Retinopathy in Rheumatic Patients with Dry Eye Symptoms
- Renal involvement in pediatric rheumatologic diseases