Yonsei Med J.
2015 Jul;56(4):1036-1043. 10.3349/ymj.2015.56.4.1036.
A Gene and Neural Stem Cell Therapy Platform Based on Neuronal Cell Type-Inducible Gene Overexpression
- Affiliations
-
- 1Department of Neurosurgery, Spine & Spinal Cord Institute and Brain Korea 21 PLUS Project for Medical Science, Yonsei University College of Medicine, Seoul, Korea. hayoon@yuhs.ac
- 2Department of Bioengineering, College of Engineering, Hanyang University, Seoul, Korea.
- KMID: 2366346
- DOI: http://doi.org/10.3349/ymj.2015.56.4.1036
Abstract
- PURPOSE
Spinal cord injury (SCI) is associated with permanent neurological damage, and treatment thereof with a single modality often does not provide sufficient therapeutic outcomes. Therefore, a strategy that combines two or more techniques might show better therapeutic effects.
MATERIALS AND METHODS
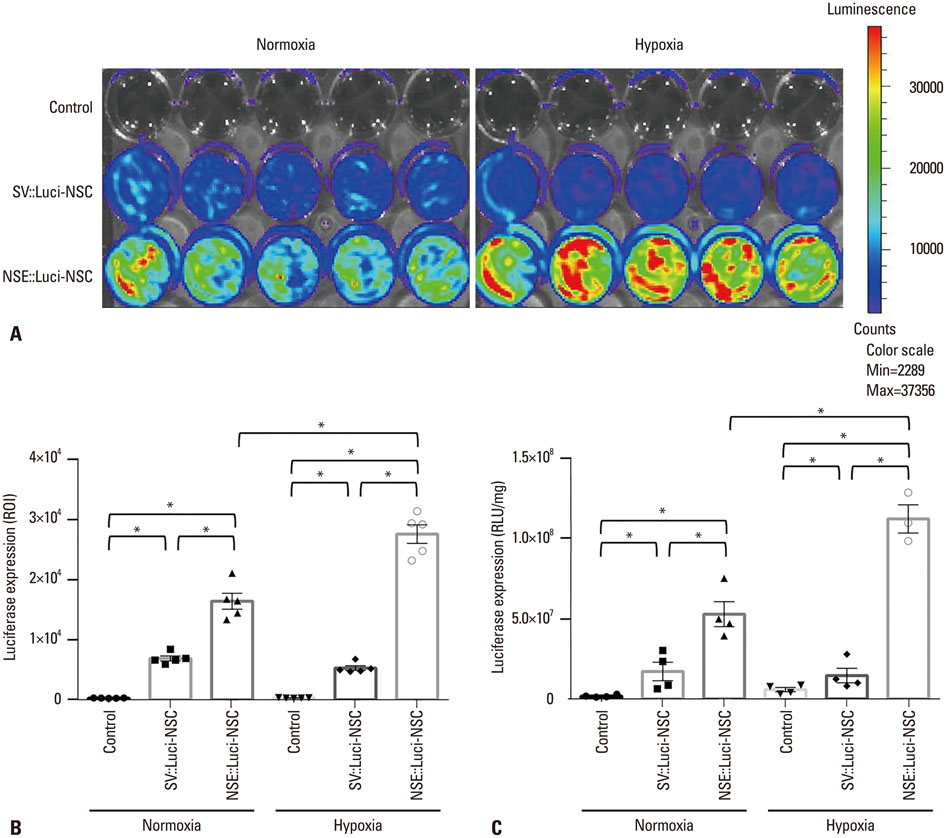
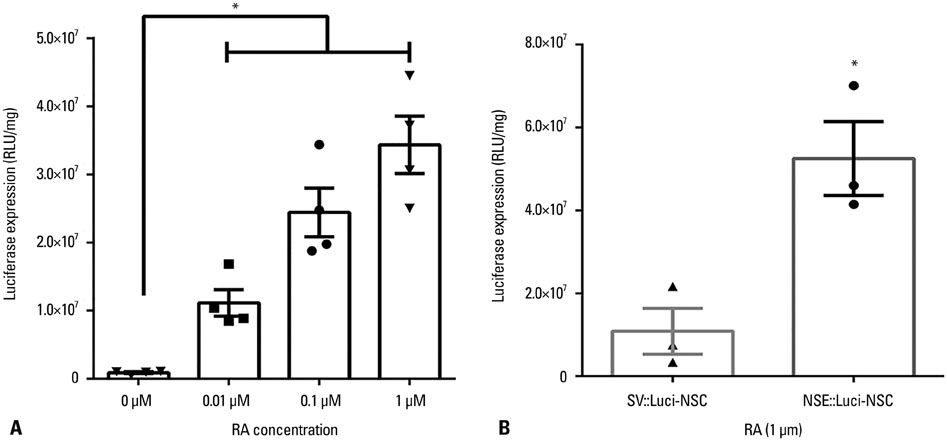
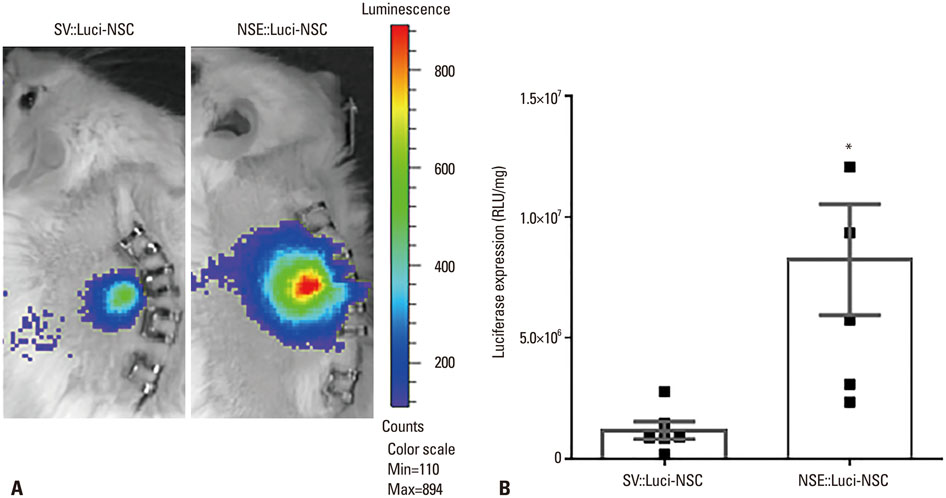
In this study, we designed a combined treatment strategy based on neural stem cells (NSCs) introduced via a neuronal cell type-inducible transgene expression system (NSE::) controlled by a neuron-specific enolase (NSE) promoter to maximize therapeutic efficiency and neuronal differentiation. The luciferase gene was chosen to confirm whether this combined system was working properly prior to using a therapeutic gene. The luciferase expression levels of NSCs introduced via the neuronal cell type-inducible luciferase expression system (NSE::Luci) or via a general luciferase expressing system (SV::Luci) were measured and compared in vitro and in vivo.
RESULTS
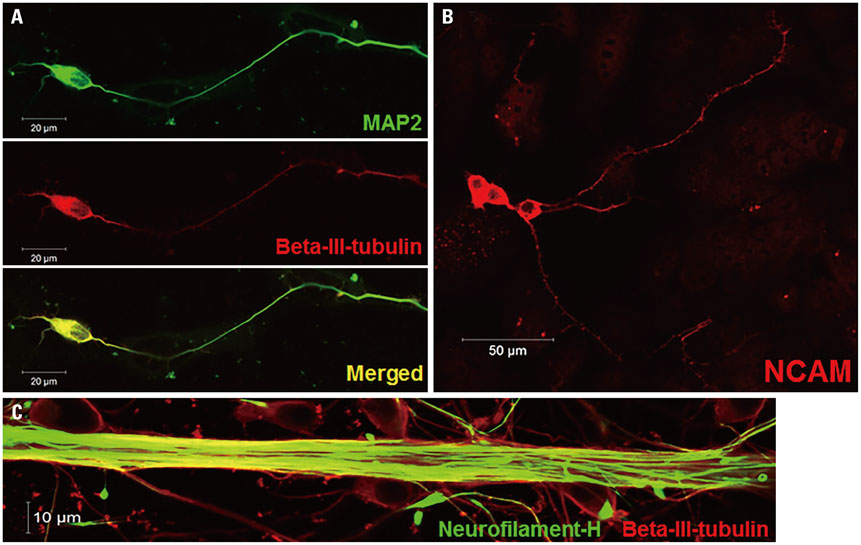
NSCs introduced via the neuronal cell type-inducible luciferase expressing system (NSE::Luci-NSCs) showed a high level of luciferase expression, compared to NSCs introduced via a general luciferase expressing system (SV::Luci-NSCs). Interestingly, the luciferase expression level of NSE::Luci-NSCs increased greatly after differentiation into neurons.
CONCLUSION
We demonstrated that a neuronal cell type-inducible gene expression system is suitable for introducing NSCs in combined treatment strategies. We suggest that the proposed strategy may be a promising tool for the treatment of neurodegenerative disorders, including SCI.
Keyword
MeSH Terms
-
Cell Differentiation/genetics/physiology
*Gene Expression
Gene Regulatory Networks
*Genetic Therapy
Humans
Luciferases/genetics/*metabolism
*Neural Stem Cells
Neurons/metabolism
Phosphopyruvate Hydratase/metabolism
Promoter Regions, Genetic
Spinal Cord Injuries/*therapy
Stem Cells/*metabolism
Luciferases
Phosphopyruvate Hydratase
Figure
Reference
-
1. Hagg T, Oudega M. Degenerative and spontaneous regenerative processes after spinal cord injury. J Neurotrauma. 2006; 23:264–280.
Article2. Lee M, Lee ES, Kim YS, Choi BH, Park SR, Park HS, et al. Ischemic injury-specific gene expression in the rat spinal cord injury model using hypoxia-inducible system. Spine (Phila Pa 1976). 2005; 30:2729–2734.
Article3. Jin H, Liu ML, Kim HA, Lee M, An S, Oh J, et al. Role of the oxygen-dependent degradation domain in a hypoxia-inducible gene expression system in vascular endothelial growth factor gene therapy. Spine (Phila Pa 1976). 2009; 34:E952–E958.
Article4. Navarro V, Millecamps S, Geoffroy MC, Robert JJ, Valin A, Mallet J, et al. Efficient gene transfer and long-term expression in neurons using a recombinant adenovirus with a neuron-specific promoter. Gene Ther. 1999; 6:1884–1892.
Article5. Tsuchiya R, Yoshiki F, Kudo Y, Morita M. Cell type-selective expression of green fluorescent protein and the calcium indicating protein, yellow cameleon, in rat cortical primary cultures. Brain Res. 2002; 956:221–229.
Article6. Kügler S, Kilic E, Bähr M. Human synapsin 1 gene promoter confers highly neuron-specific long-term transgene expression from an adenoviral vector in the adult rat brain depending on the transduced area. Gene Ther. 2003; 10:337–347.
Article7. Iwai H, Nori S, Nishimura S, Yasuda A, Takano M, Tsuji O, et al. Transplantation of neural stem/progenitor cells at different locations in mice with spinal cord injury. Cell Transplant. 2014; 23:1451–1464.
Article8. Lu P, Wang Y, Graham L, McHale K, Gao M, Wu D, et al. Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell. 2012; 150:1264–1273.
Article9. Liu ML, Oh JS, An SS, Pennant WA, Kim HJ, Gwak SJ, et al. Controlled nonviral gene delivery and expression using stable neural stem cell line transfected with a hypoxia-inducible gene expression system. J Gene Med. 2010; 12:990–1001.
Article10. Kim HM, Hwang DH, Lee JE, Kim SU, Kim BG. Ex vivo VEGF delivery by neural stem cells enhances proliferation of glial progenitors, angiogenesis, and tissue sparing after spinal cord injury. PLoS One. 2009; 4:e4987.
Article11. Kim HJ, Oh JS, An SS, Pennant WA, Gwak SJ, Kim AN, et al. Hypoxia-specific GM-CSF-overexpressing neural stem cells improve graft survival and functional recovery in spinal cord injury. Gene Ther. 2012; 19:513–521.
Article12. Oh JS, An SS, Gwak SJ, Pennant WA, Kim KN, Yoon DH, et al. Hypoxia-specific VEGF-expressing neural stem cells in spinal cord injury model. Neuroreport. 2012; 23:174–178.
Article13. An SS, Jin HL, Kim KN, Kim DS, Cho J, Liu ML, et al. Neuroprotective effect of combined hypoxia-induced VEGF and bone marrow-derived mesenchymal stem cell treatment. Childs Nerv Syst. 2010; 26:323–331.
Article14. Lu P, Jones LL, Snyder EY, Tuszynski MH. Neural stem cells constitutively secrete neurotrophic factors and promote extensive host axonal growth after spinal cord injury. Exp Neurol. 2003; 181:115–129.
Article