Yonsei Med J.
2015 Jul;56(4):944-950. 10.3349/ymj.2015.56.4.944.
Blood Mercury and Insulin Resistance in Nondiabetic Koreans (KNHANES 2008-2010)
- Affiliations
-
- 1Department of Family Practice and Community Health, Ajou University School of Medicine, Suwon, Korea. jchcmc@hanmail.net
- 2CHA Antiaging Institute, CHA University, Seoul, Korea.
- KMID: 2366333
- DOI: http://doi.org/10.3349/ymj.2015.56.4.944
Abstract
- PURPOSE
Blood mercury levels are associated with inflammation, and chronic low-grade inflammation is a cause of insulin resistance. This study aimed to investigate the association between serum mercury and insulin resistance.
MATERIALS AND METHODS
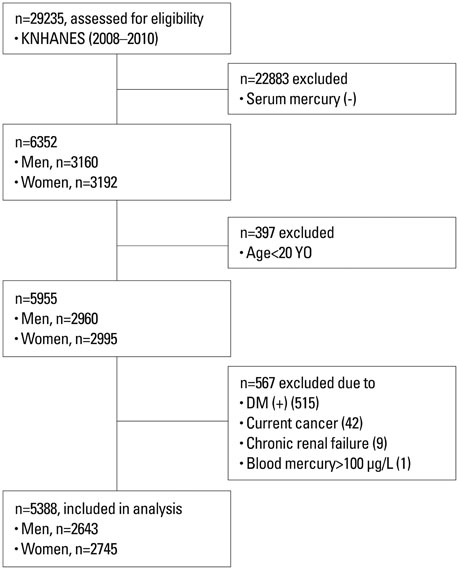
Subjects from the 2008-2010 Korean National Health and Nutrition Examination Survey were selected (n=29235) and the relevant data of 5388 subjects (2643 males and 2745 females) were analyzed cross-sectionally. Homeostasis Model Assessment for Insulin Resistance (HOMA-IR) was compared according to blood mercury quartiles, and the odds ratio (OR) of having the highest quartile of HOMA-IR according to blood mercury quartiles was calculated.
RESULTS
Blood mercury levels in men and women were 29.4 nmol/L and 20.5 nmol/L, respectively, and fasting blood sugar (FBS), insulin, and HOMA-IR were significantly correlated with blood mercury levels. The correlation was stronger in men than in women. In men, FBS and HOMA-IR showed step-wise increases as the quartiles of blood mercury increased; only HOMA-IR differed significantly in the third and fourth blood mercury quartiles, compared to the first quartile. In women, however, both FBS and HOMA-IR differed significantly in the third and fourth blood mercury quartiles, compared to the first quartile. Among men, the OR of being in the highest HOMA-IR quartile was greatest for the highest blood mercury quartile (OR=1.720, 95% CI; 1.172-2.526), compared with the lowest quartile.
CONCLUSION
In this large population-based study, blood mercury levels were weakly correlated with HOMA-IR and may be a risk factor for insulin resistance in nondiabetic Koreans.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Association between Blood Mercury Level and Visceral Adiposity in Adults
Jong Suk Park, Kyoung Hwa Ha, Ka He, Dae Jung Kim
Diabetes Metab J. 2017;41(2):113-120. doi: 10.4093/dmj.2017.41.2.113.
Reference
-
1. Romeo GR, Lee J, Shoelson SE. Metabolic syndrome, insulin resistance, and roles of inflammation--mechanisms and therapeutic targets. Arterioscler Thromb Vasc Biol. 2012; 32:1771–1776.
Article2. Savoia C, Schiffrin EL. Inflammation in hypertension. Curr Opin Nephrol Hypertens. 2006; 15:152–158.
Article3. Boos CJ, Lip GY. Is hypertension an inflammatory process? Curr Pharm Des. 2006; 12:1623–1635.
Article4. Meigs JB, Rutter MK, Sullivan LM, Fox CS, D'Agostino RB Sr, Wilson PW. Impact of insulin resistance on risk of type 2 diabetes and cardiovascular disease in people with metabolic syndrome. Diabetes Care. 2007; 30:1219–1225.
Article5. Lee JM, Okumura MJ, Davis MM, Herman WH, Gurney JG. Prevalence and determinants of insulin resistance among U S adolescents: a population-based study. Diabetes Care. 2006; 29:2427–2432.6. Chen G, Liu C, Yao J, Jiang Q, Chen N, Huang H, et al. Overweight, obesity, and their associations with insulin resistance and β-cell function among Chinese: a cross-sectional study in China. Metabolism. 2010; 59:1823–1832.
Article7. Lee IT, Lee WJ, Huang CN, H-H Sheu W. The association of low-grade inflammation, urinary albumin, and insulin resistance with metabolic syndrome in nondiabetic Taiwanese. Metabolism. 2007; 56:1708–1713.
Article8. Turunen AW, Männistö S, Kiviranta H, Marniemi J, Jula A, Tiittanen P, et al. Dioxins, polychlorinated biphenyls, methyl mercury and omega-3 polyunsaturated fatty acids as biomarkers of fish consumption. Eur J Clin Nutr. 2010; 64:313–323.
Article9. Clarkson TW, Magos L, Myers GJ. The toxicology of mercury--current exposures and clinical manifestations. N Engl J Med. 2003; 349:1731–1737.
Article10. Mozaffarian D, Rimm EB. Fish intake, contaminants, and human health: evaluating the risks and the benefits. JAMA. 2006; 296:1885–1899.11. Houston MC. Role of mercury toxicity in hypertension, cardiovascular disease, and stroke. J Clin Hypertens (Greenwich). 2011; 13:621–627.
Article12. Chang JW, Chen HL, Su HJ, Liao PC, Guo HR, Lee CC. Simultaneous exposure of non-diabetics to high levels of dioxins and mercury increases their risk of insulin resistance. J Hazard Mater. 2011; 185:749–755.
Article13. Lund BO, Miller DM, Woods JS. Studies on Hg(II)-induced H2O2 formation and oxidative stress in vivo and in vitro in rat kidney mitochondria. Biochem Pharmacol. 1993; 45:2017–2024.
Article14. Salonen JT, Seppänen K, Nyyssönen K, Korpela H, Kauhanen J, Kantola M, et al. Intake of mercury from fish, lipid peroxidation, and the risk of myocardial infarction and coronary, cardiovascular, and any death in eastern Finnish men. Circulation. 1995; 91:645–655.
Article15. Lee S, Choi S, Kim HJ, Chung YS, Lee KW, Lee HC, et al. Cutoff values of surrogate measures of insulin resistance for metabolic syndrome in Korean non-diabetic adults. J Korean Med Sci. 2006; 21:695–700.
Article16. Jomova K, Valko M. Advances in metal-induced oxidative stress and human disease. Toxicology. 2011; 283:65–87.
Article17. Philibert A, Bouchard M, Mergler D. Neuropsychiatric symptoms, omega-3, and mercury exposure in freshwater fish-eaters. Arch Environ Occup Health. 2008; 63:143–153.
Article18. Lim C. Korea Health Statistics 2011: Korea National Health and Nutrition Examination Survey (KNHANES V-2). Seoul, Korea: Welfare MoH;2012.19. McKelvey W, Gwynn RC, Jeffery N, Kass D, Thorpe LE, Garg RK, et al. A biomonitoring study of lead, cadmium, and mercury in the blood of New York city adults. Environ Health Perspect. 2007; 115:1435–1441.
Article20. Kim NS, Lee BK. National estimates of blood lead, cadmium, and mercury levels in the Korean general adult population. Int Arch Occup Environ Health. 2011; 84:53–63.
Article21. Vupputuri S, Longnecker MP, Daniels JL, Guo X, Sandler DP. Blood mercury level and blood pressure among US women: results from the National Health and Nutrition Examination Survey 1999-2000. Environ Res. 2005; 97:195–200.
Article22. Fillion M, Mergler D, Sousa Passos CJ, Larribe F, Lemire M, Guimarães JR. A preliminary study of mercury exposure and blood pressure in the Brazilian Amazon. Environ Health. 2006; 5:29.
Article23. Yeap BB, Chubb SA, Hyde Z, Jamrozik K, Hankey GJ, Flicker L, et al. Lower serum testosterone is independently associated with insulin resistance in non-diabetic older men: the Health In Men Study. Eur J Endocrinol. 2009; 161:591–598.
Article24. Kang ES, Yun YS, Park SW, Kim HJ, Ahn CW, Song YD, et al. Limitation of the validity of the homeostasis model assessment as an index of insulin resistance in Korea. Metabolism. 2005; 54:206–211.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Cut-off Value of Blood Mercury Concentration in Relation to Insulin Resistance
- Comparison of insulin resistance and serum hsCRP levels according to the fasting blood glucose and blood pressure in nondiabetic and normotensive range
- Insulin Resistance Syndrome in Koreans
- Blood Heavy Metal Concentrations of Korean Adults by Seafood Consumption Frequency: Using the Fourth Korea National Health and Nutrition Examination Survey (KNHANES IV), 2008
- Association between Smoking Status and Insulin Resistance in Apparently Healthy Korean Men