J Dent Anesth Pain Med.
2016 Dec;16(4):253-261. 10.17245/jdapm.2016.16.4.253.
Developmental procedures for the clinical practice guidelines for conscious sedation in dentistry for the Korean Academy of Dental Sciences
- Affiliations
-
- 1Department of Pediatric Dentistry, College of Dentistry, Wonkwang University, Daejeon, Korea.
- 2Department of Dental Anesthesiology and Dental Research Institute, School of Dentistry, Seoul National University, Seoul, Korea. dentane@snu.ac.kr
- 3Department of Dental Anesthesiology, School of Dentistry, Dankook University, Cheonan, Korea.
- 4Department of Pediatric Dentistry, School of Dentistry, Dankook University, Cheonan, Korea.
- 5Department of Oral and Maxillofacial surgery, Kyung Hee University Dental Hospital at Gangdong, Kyung Hee University, Seoul, Korea.
- 6Department of Dentistry/Oral & Maxillofacial Surgery, College of Medicine, Hanyang University, Seoul, Korea.
- KMID: 2366153
- DOI: http://doi.org/10.17245/jdapm.2016.16.4.253
Abstract
- BACKGROUND
Evidence-based clinical practice guidelines (CPGs) are defined as "statements that are scientifically reviewed about evidence and systematically developed to assist in the doctors' and patients' decision making in certain clinical situations." This recommendation aims to promote good clinical practice for the provision of safe and effective practices of conscious sedation in dentistry.
METHODS
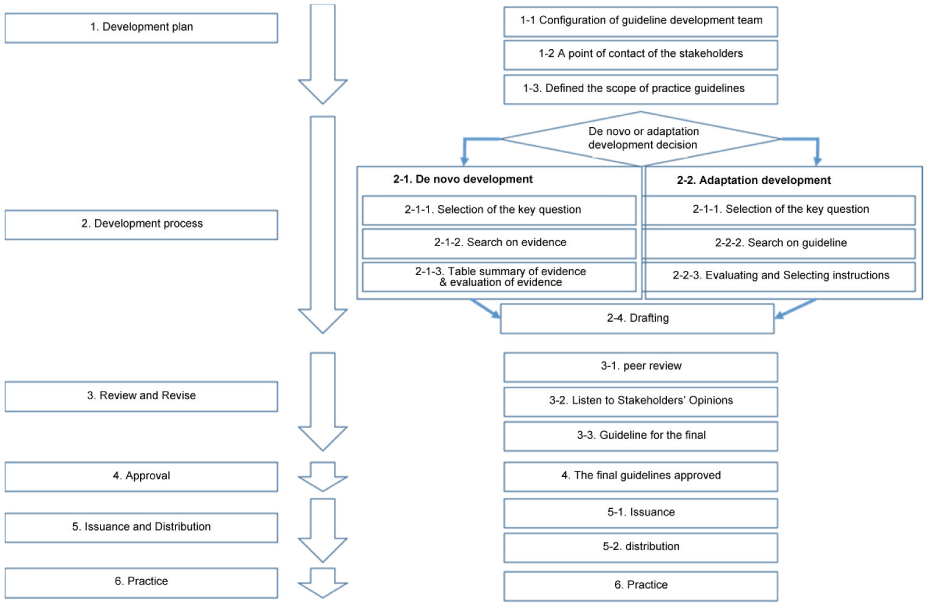
The development of this clinical practice guideline was conducted by performing a systematic search of the literature for evidence-based CPGs. Existing guidelines, relevant systematic reviews, policy documents, legislation, or other recommendations were reviewed and appraised. To supplement this information, key questions were formulated by the Guideline Development Group and used as the basis for designing systematic literature search strategies to identify literature that may address these questions. Guideline documents were evaluated through a review of domestic and international databases for the development of a renewing of existing conscious sedation guidelines for dentistry. Clinical practice guidelines were critically appraised for their methodologies using Appraisal of guidelines for research and evaluation (AGREE) II.
RESULTS
A total of 12 existing CPGs were included and 13 recommendations were made in a range of general, adult, and pediatric areas.
CONCLUSION
The clinical practice guidelines for conscious sedation will be reviewed in 5 years' time for further updates to reflect significant changes in the field.
Figure
Cited by 1 articles
-
The effect of dental scaling noise during intravenous sedation on acoustic respiration rate (RRa™)
Jung Ho Kim, Seong In Chi, Hyun Jeong Kim, Kwang-Suk Seo
J Dent Anesth Pain Med. 2018;18(2):97-103. doi: 10.17245/jdapm.2018.18.2.97.
Reference
-
1. Committee to advise the public health service on clinical practice guidelines, Institute of Medicine. Field MJ, Lohr KN, editors. Clinical practice guidelines: directions for a new program. Washington DC: National Academy Press;1990.
Article2. Committee on Standards for Developing Trustworthy Clinical Practice Guidelines, Board on Health Care Services, Institute of Medicine. Graham R, Mancher M, Wolman DM, Greenfield S, Steinberg E, editors. Clinical practice guidelines we can trust. Washington DC: National Academy Press;2011.
Article3. Ji SM, Kim SY, Shin SS, Heo DS, Kim NS. Consensus on definition and quality standard of clinical practice guideline using RAND method. Korean J Health Policy Adm. 2010; 20:1–16.4. American Dental Association. Guidelines for the use of sedation and general anesthesia by dentists, 2007. 2012.5. Evidence-Based Medicine Working Group. Evidence-based medicine. A new approach to teaching the practice of medicine. JAMA. 1992; 268:2420.6. Nair R, Aggarwal R, Khanna D. Methods of formal consensus in classification/diagnostic criteria and guideline development. Seminars in arthritis and rheumatism. WB Saunders;2011.7. Rycroft-Malone J. Formal consensus: the development of a national clinical guideline. Qual Health Care. 2001; 10:238–244.8. Robinson N, Liu J, Lee MS. Clinical guidelines: the way for best practice. Eur J Integr Med. 2014; 2:133–134.9. Kim S, Jee S, Lee S, Lee Y, Park J, Nam M. Guidance for development of clinical practice guidelines. National Evidence-based Healthcare Collaborating Agency;2011. p. 20–77.
Article10. Korean Academy of Dental Sciences. 2010 Guidelines for the Use of Sedation by Dentists. Koonja publishing Inc;2010. 2014 Feb 21. Available from http://www.kadents.or.kr/modules/bbs/index.php?code=pds&mode=view&id=6&___M_ID=100.
Article11. Littlejohns P, Thomason M, Cluzeau F. Guideline development in Europe-An international comparison. Int J Technol Assess Health Care. 2000; 16:1039–1049.
Article12. Turner T, Misso M, Harris C, Green S. Development of evidence-based clinical practice guidelines (CPGs): comparing approaches. Implement Sci. 2008; 3:1.
Article13. Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, et al. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010; 182:E839–E842.14. Cho HS, Shin IS, Oh MK, Lee YK, Kim JK, Jung YM, et al. A Strategy for Dissemination and Implementation of Clinical Practice Guidelines in Korea. National Evidence-based Healthcare Collaborating Agency;2014.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Current trends in intravenous sedative drugs for dental procedures
- Midazolam use in pediatric dentistry: a review
- Sedation of the Pediatric Dental Patient
- Trends of conscious sedation in the Department of Pediatric Dentistry at the Dankook University Dental Hospital for 11 Years
- Sedation at Sedation Clinic of Department of Dentistry in Hanyang University Medical Center (II)