Anat Cell Biol.
2016 Dec;49(4):231-240. 10.5115/acb.2016.49.4.231.
Descent of mesonephric duct to the final position of the vas deferens in human embryo and fetus
- Affiliations
-
- 1Department of Anatomy, Histology and Embryology, Yanbian University Medical College, Yanji, China. zwjin@ybu.edu.cn
- 2Department of Anatomy, Akita University School of Medicine, Akita, Japan.
- 3Department of Urology, Kobe University School of Medicine, Kobe, Japan.
- 4Division of Internal Medicine, Iwamizawa Asuka Hospital, Iwamizawa, Japan.
- 5Department of Anatomy and Human Embryology, Institute of Embryology, Faculty of Medicine, Complutense University, Madrid, Spain.
- KMID: 2365552
- DOI: http://doi.org/10.5115/acb.2016.49.4.231
Abstract
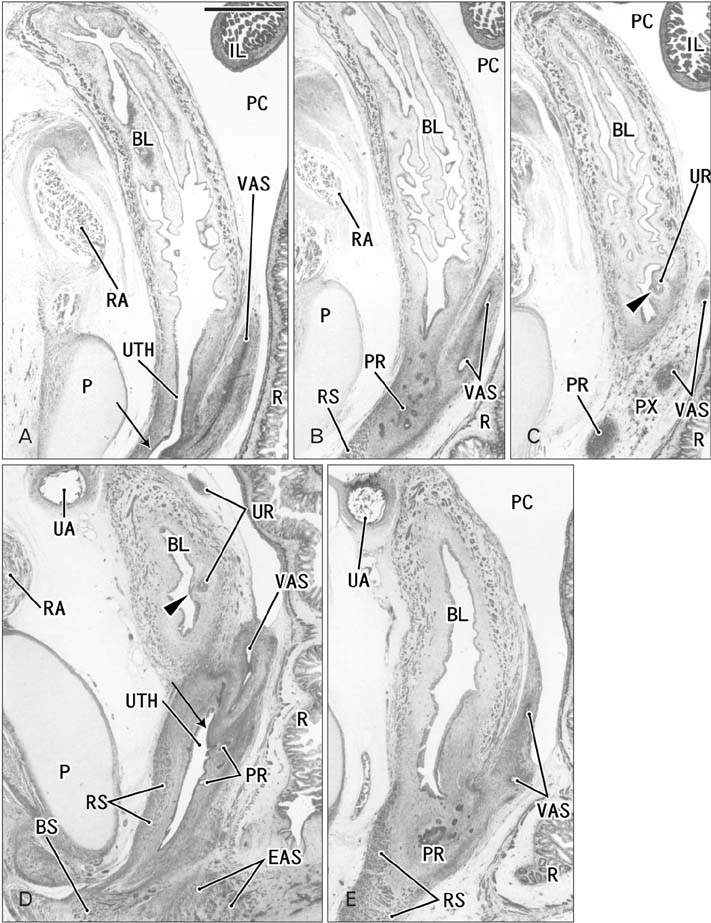
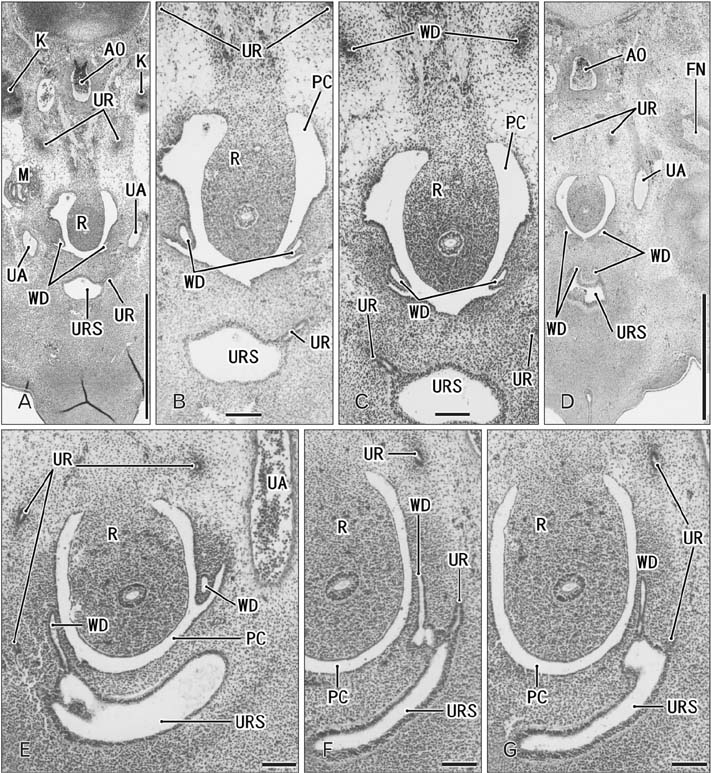
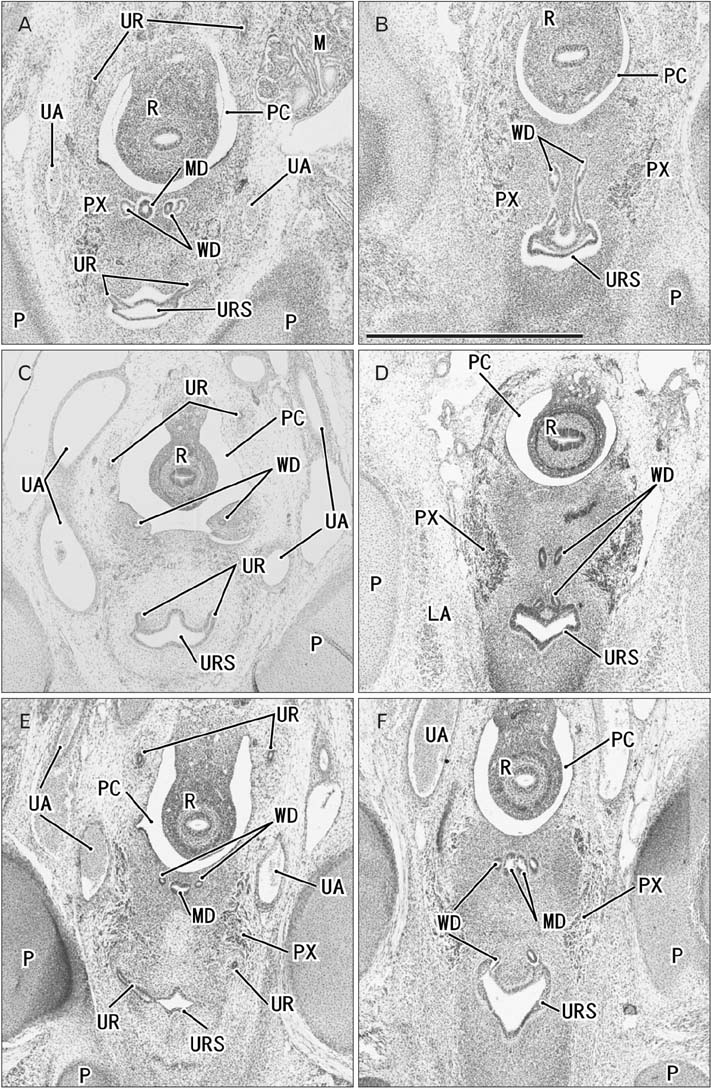
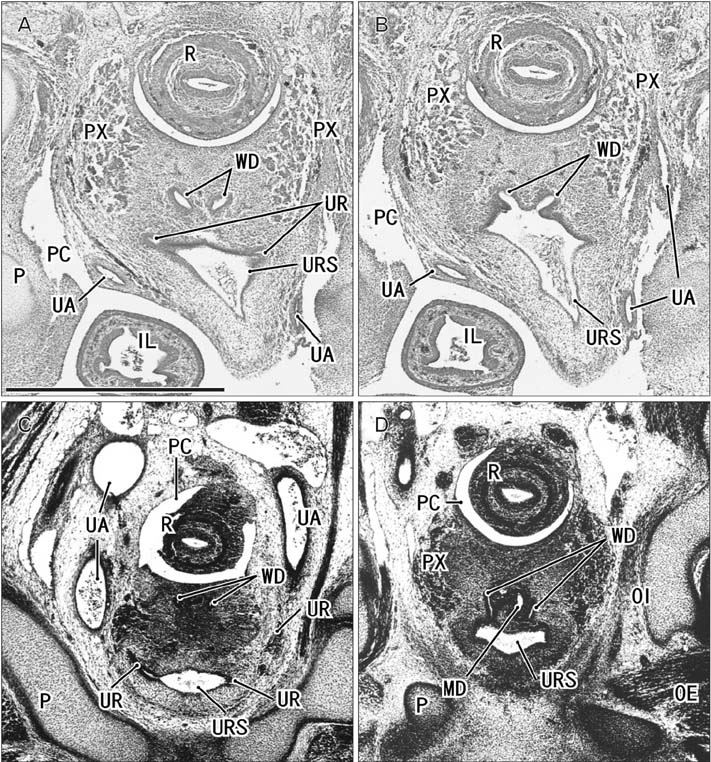
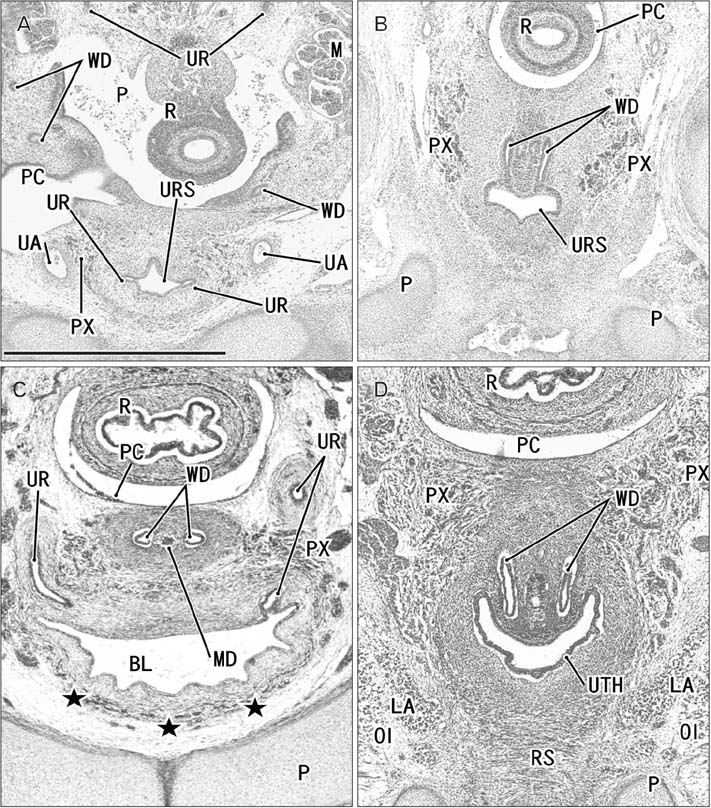
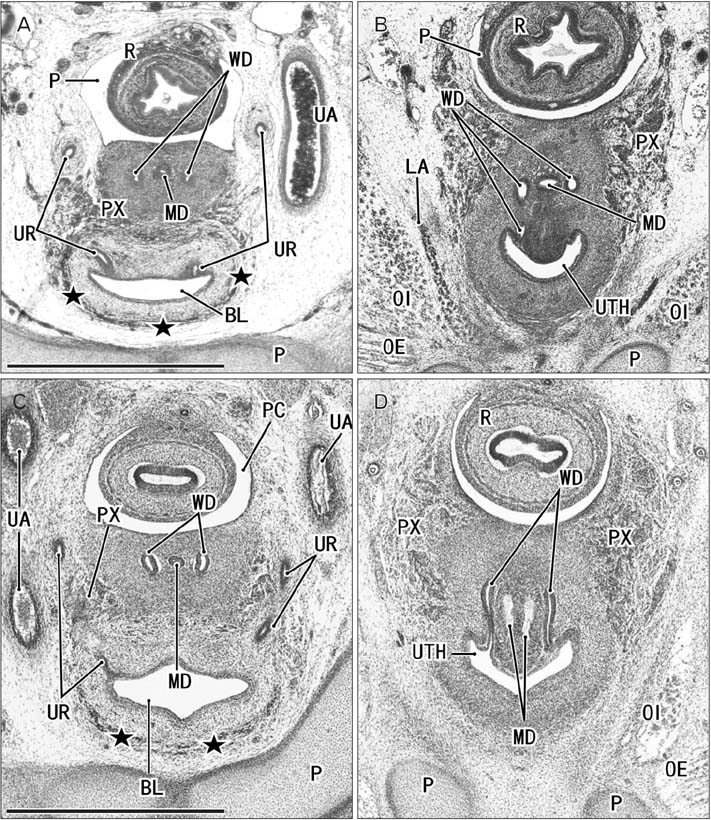
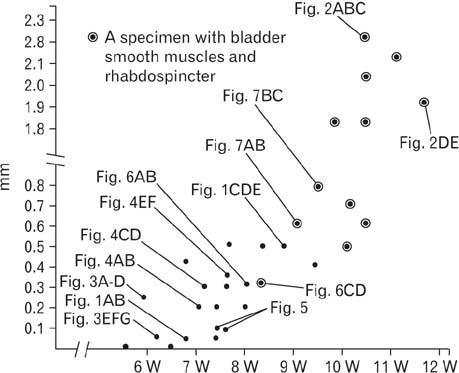
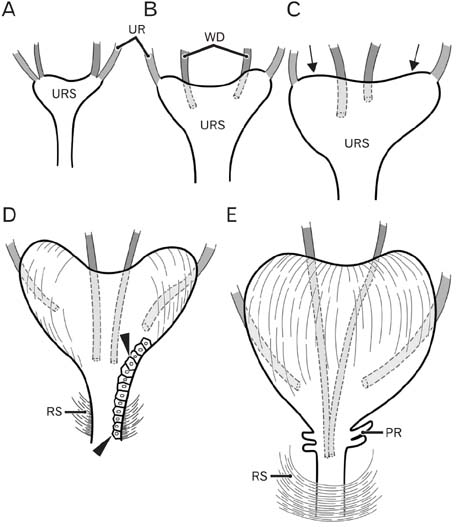
- Because the ureter arises from the mesonephric or Wolffian duct (WD), the WD opening should migrate inferiorly along the urogenital sinus or future urethra. However, this process of descent has not been evaluated morphometrically in previous studies and we know little about intermediate morphologies for the descent. In the present work, serial sagittal sections of 15 specimens at gestational age 6-12 weeks and serial horizontal sections of 20 specimens at 6-10 weeks were analyzed. Monitoring of horizontal sections showed that, until 9 weeks, a heart-, lozenge- or oval-shape of the initial urogenital sinus remained in the bladder and urethra. Thus, the future bladder and urethra could not be distinguished by the transverse section or plane. The maximum width of the urogenital sinus or bladder at 6-10 weeks was 0.8 mm, although its supero-inferior length reached 5 mm at 10 weeks. During earlier stages, however, the medial shift of the WD was rather evident. Depending on the extent of upward growth of the bladder smooth muscle, the descent of the vas deferens became evident at 10-12 weeks. Development of the urethral rhabdosphincter likely resulted in the differentiation of urogenital sinus into the urethra and bladder before formation of the bladder neck with 3-layered smooth muscles. Development of the prostate followed these morphological changes, later accelerating the further descent of the WD opening. Because of their close topographical relationships, slight anomalies or accidents of the umbilical cord at 10-12 weeks may have a significant effect on normal anatomy.
MeSH Terms
Figure
Reference
-
1. Hamilton WJ, Mossman HW. Human embryology. 4th ed. London: Williams & Wilkins;1978. p. 321–322.2. O'Rhahilly R, Műller F. Human embryology and teratology. 2nd ed. New York: Wiley-Liss;1996. p. 194–201.3. Moore KL, Persaud TV. The developing human: clinically oriented embryology. 6th ed. Philadelphia: Saunders;1998. p. 244–260.4. Naito M, Hinata N, Rodriguez-Vazquez JF, Murakami G, Aizawa S, Fujisawa M. Absorption of the Wolffian duct and duplicated ureter by the urogenital sinus: morphological study using human fetuses and embryos. BJU Int. 2015; 116:135–141.5. Masumoto H, Rodríguez-Vázquez JF, Verdugo-López S, Murakami G, Matsubara A. Fetal topographical anatomy of the female urethra and descending vagina: a histological study of the early human fetal urethra. Ann Anat. 2011; 193:500–508.6. Mendelsohn C. Using mouse models to understand normal and abnormal urogenital tract development. Organogenesis. 2009; 5:306–314.7. Tanaka ST, Ishii K, Demarco RT, Pope JC 4th, Brock JW 3rd, Hayward SW. Endodermal origin of bladder trigone inferred from mesenchymal-epithelial interaction. J Urol. 2010; 183:386–391.8. Evatt EJ. A contribution to the development of the prostate gland in the human female, and a study of the homologies of the urethra and vagina of the sexes. J Anat Physiol. 1911; 45(Pt 2):122–130.9. Parrott TS, Gray SW, Skandalakis JE. Bladder and urethra. In : Skandalakis JE, Gray SW, editors. Embryology for Surgeons. 2nd ed. Baltimore: Williams & Wilkins;1994. p. 671–717.10. Suson KD, Novak TE, Gupta AD, Benson J, Sponseller P, Gearhart JP. Neuro-orthopedic manifestations of the omphalocele exstrophy imperforate anus spinal defects complex. J Urol. 2010; 184:4 Suppl. 1651–1655.11. Kim JH, Hwang SE, Rodríguez-Vázquez JF, Murakami G, Cho BH. Liver agenesis with omphalocele: a report of two human embryos using serial histological sections. Pediatr Dev Pathol. 2014; 17:431–440.12. Fuchs F, Picone O, Levaillant JM, Mabille M, Mas AE, Frydman R, Senat MV. Prenatal diagnosis of a patent urachus cyst with the use of 2D, 3D, 4D ultrasound and fetal magnetic resonance imaging. Fetal Diagn Ther. 2008; 24:444–447.13. Bischoff A, Calvo-Garcia MA, Baregamian N, Levitt MA, Lim FY, Hall J, Peña A. Prenatal counseling for cloaca and cloacal exstrophy-challenges faced by pediatric surgeons. Pediatr Surg Int. 2012; 28:781–788.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Duplicated Vas Deferens Found Incidentally during Varicocelectomy
- A Case Report of Bilateral Absence of Vas Deferens
- Effect of antiandrogens upon the morphological development of gubernaculum and the testicular descent in rats
- Vas anomaly associated with ipsilateral renal hypoplasia
- Pharmacological Effects on Vas Deferens