Allergy Asthma Immunol Res.
2015 Mar;7(2):124-129. 10.4168/aair.2015.7.2.124.
In Vitro Evaluation of Allergen Potencies of Commercial House Dust Mite Sublingual Immunotherapy Reagents
- Affiliations
-
- 1Department of Internal Medicine and Institute of Allergy, Yonsei University College of Medicine, Seoul, Korea. parkjw@yuhs.ac
- 2Center for Immunology and Pathology, Korea National Institute of Health, Osong, Korea.
- KMID: 2365540
- DOI: http://doi.org/10.4168/aair.2015.7.2.124
Abstract
- PURPOSE
The clinical efficacy of allergen-immunotherapy is known to be dose dependent. However, optimal maintenance dosage has not yet been determined for sublingual immunotherapy (SLIT). Furthermore, since companies adopt their own units for expression of allergenicity, the allergen concentrations of individual reagents cannot be compared easily. We sought to measure and compare the allergenicities of 3 commercially available house dust mite (HDM) SLIT regents and a subcutaneous immunotherapy reagent.
METHODS
We measured the HDM allergenic potency of the maintenance dosages of three SLIT reagents: Staloral(R) (300 index of reactivity [IR] /mL, recommended maintenance dosage [MD]: 120 IR), SLITone(R) (1,000 standard therapeutic unit [STU]/mL, recommended MD: 200 STU), Wolwopharma(R) (100 microg/mL, recommended MD: 20 microg), and subcutaneous immunotherapy regents of Hollister-Stier (10,000 allergy unit [AU] /mL). The allergenic potency was assessed by measuring the total protein concentrations, mite group 1 and 2 allergens using 2-site ELISA, and an inhibition test against IgE specific to Dermatophagoides farinae and Dermatophagoides pteronyssinus.
RESULTS
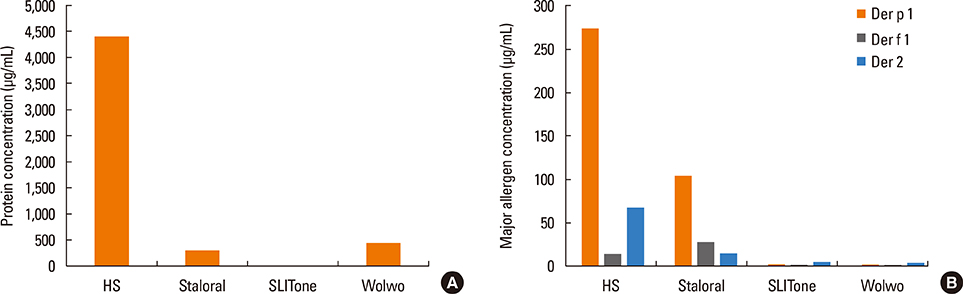
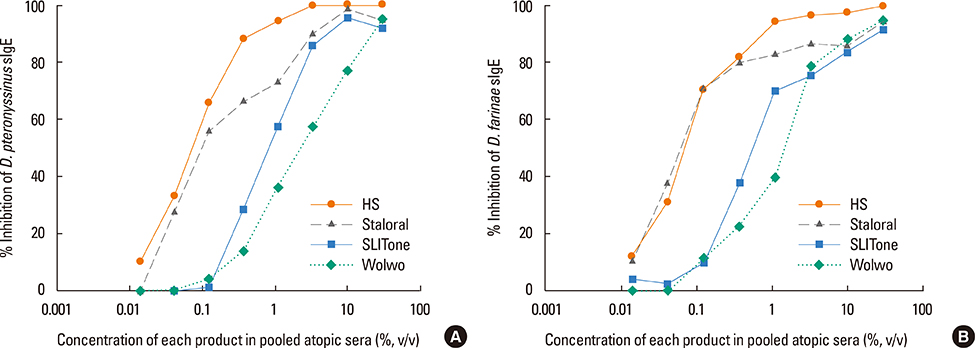
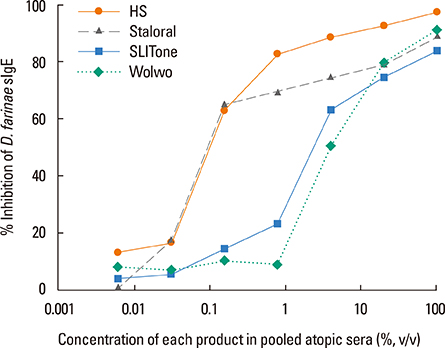
The protein content of the Wolwopharma(R) reagent was 1.5-261.4 times higher than that of the other 2 SLIT reagents. The concentration of group 1 major allergens in Staloral(R) (132.03 microg/mL) was 33- to 44.5-fold higher than in SLITone(R) (4.00 microg/mL) and Wolwopharma(R) (2.97 microg/mL). The concentration of group 2 major allergen was also 8.9- to 10.5-fold higher in Staloral(R) (15.7 microg/mL) than in SLITone(R) (1.8 microg/mL) or Wolwopharma(R) (1.5 microg/mL). An ELISA inhibition study against HDM-specific IgE showed that the allergen potency of Staloral(R) reagent is 8.5-fold and 21-fold higher than that of SLITone(R) or Wolwopharma(R), respectively. The differences between the maintenance dosages are further exaggerated by the differences in the recommended volumes of SLIT reagents.
CONCLUSIONS
The allergen potencies of commercially available HDM SLIT reagents are markedly different. Consensus regarding the optimal allergen concentration for SLIT reagents used to treat HDM respiratory allergies is needed.
MeSH Terms
Figure
Reference
-
1. Radulovic S, Wilson D, Calderon M, Durham S. Systematic reviews of sublingual immunotherapy (SLIT). Allergy. 2011; 66:740–752.2. Compalati E, Passalacqua G, Bonini M, Canonica GW. The efficacy of sublingual immunotherapy for house dust mites respiratory allergy: results of a GA2LEN meta-analysis. Allergy. 2009; 64:1570–1579.3. Canonica GW, Bousquet J, Casale T, Lockey RF, Baena-Cagnani CE, Pawankar R, et al. Sub-lingual immunotherapy: World Allergy Organization Position Paper 2009. Allergy. 2009; 64:Suppl 91. 1–59.4. Hur GY, Kim TB, Han MY, Nahm DH, Park JW. Allergen and Immunotherapy Work Group of the Korean Academy of Asthma, Allergy and Clinical Immunology (KAAACI). A survey of the prescription patterns of allergen immunotherapy in Korea. Allergy Asthma Immunol Res. 2013; 5:277–282.5. Calderón MA, Simons FE, Malling HJ, Lockey RF, Moingeon P, Demoly P. Sublingual allergen immunotherapy: mode of action and its relationship with the safety profile. Allergy. 2012; 67:302–311.6. Norman PS. An overview of immunotherapy: implications for the future. J Allergy Clin Immunol. 1980; 65:87–96.7. Joint Task Force on Practice Parameters. American Academy of Allergy, Asthma and Immunology. American College of Allergy, Asthma and Immunology. Joint Council of Allergy, Asthma and Immunology. Allergen immunotherapy: a practice parameter second update. J Allergy Clin Immunol. 2007; 120:S25–S85.8. Ewbank PA, Murray J, Sanders K, Curran-Everett D, Dreskin S, Nelson HS. A double-blind, placebo-controlled immunotherapy dose-response study with standardized cat extract. J Allergy Clin Immunol. 2003; 111:155–161.9. Durham SR, Yang WH, Pedersen MR, Johansen N, Rak S. Sublingual immunotherapy with once-daily grass allergen tablets: a randomized controlled trial in seasonal allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2006; 117:802–809.10. Didier A, Malling HJ, Worm M, Horak F, Jäger S, Montagut A, et al. Optimal dose, efficacy, and safety of once-daily sublingual immunotherapy with a 5-grass pollen tablet for seasonal allergic rhinitis. J Allergy Clin Immunol. 2007; 120:1338–1345.11. Bousquet J, Bieber T, Fokkens W, Humbert M, Kowalski ML, Niggemann B, et al. Consensus statements, evidence-based medicine and guidelines in allergic diseases. Allergy. 2008; 63:1–4.12. Mösges R, Pasch N, Schlierenkämper U, Lehmacher W. Comparison of the biological activity of the most common sublingual allergen solutions made by two European manufacturers. Int Arch Allergy Immunol. 2006; 139:325–329.13. Larenas-Linnemann D, Esch R, Plunkett G, Brown S, Maddox D, Barnes C, et al. Maintenance dosing for sublingual immunotherapy by prominent European allergen manufacturers expressed in bioequivalent allergy units. Ann Allergy Asthma Immunol. 2011; 107:448–458.14. Cox L, Jacobsen L. Comparison of allergen immunotherapy practice patterns in the United States and Europe. Ann Allergy Asthma Immunol. 2009; 103:451–459.15. Larenas-Linnemann D, Cox LS. Immunotherapy and Allergy Diagnostics Committee of the American Academy of Allergy, Asthma and Immunology. European allergen extract units and potency: review of available information. Ann Allergy Asthma Immunol. 2008; 100:137–145.16. Bush RK, Swenson C, Fahlberg B, Evans MD, Esch R, Morris M, et al. House dust mite sublingual immunotherapy: results of a US trial. J Allergy Clin Immunol. 2011; 127:974–981.e1-7.17. Slater JE, Pastor RW. The determination of equivalent doses of standardized allergen vaccines. J Allergy Clin Immunol. 2000; 105:468–474.18. Cox L, Esch RE, Corbett M, Hankin C, Nelson M, Plunkett G. Allergen immunotherapy practice in the United States: guidelines, measures, and outcomes. Ann Allergy Asthma Immunol. 2011; 107:289–299.19. Francis JN, Durham SR. Adjuvants for allergen immunotherapy: experimental results and clinical perspectives. Curr Opin Allergy Clin Immunol. 2004; 4:543–548.20. van Ree R. Indoor allergens: relevance of major allergen measurements and standardization. J Allergy Clin Immunol. 2007; 119:270–277.21. Jeong KY, Hong CS, Lee JS, Park JW. Optimization of allergen standardization. Yonsei Med J. 2011; 52:393–400.22. Park JW, Kim KS, Jin HS, Kim CW, Kang DB, Choi SY, et al. Der p 2 isoallergens have different allergenicity, and quantification with 2-site ELISA using monoclonal antibodies is influenced by the isoallergens. Clin Exp Allergy. 2002; 32:1042–1047.23. van Ree R, Chapman MD, Ferreira F, Vieths S, Bryan D, Cromwell O, et al. The CREATE project: development of certified reference materials for allergenic products and validation of methods for their quantification. Allergy. 2008; 63:310–326.24. Chapman MD, Ferreira F, Villalba M, Cromwell O, Bryan D, Becker WM, et al. The European Union CREATE project: a model for international standardization of allergy diagnostics and vaccines. J Allergy Clin Immunol. 2008; 122:882–889.e2.25. Bousquet J, Lockey R, Malling HJ. Allergen immunotherapy: therapeutic vaccines for allergic diseases. A WHO position paper. J Allergy Clin Immunol. 1998; 102:558–562.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Erratum: In Vitro Evaluation of Allergen Potencies of Commercial House Dust Mite Sublingual Immunotherapy Reagents
- New approaches to immunotherapy in house dust mite allergy
- Effect on quality of life of the mixed house dust mite/weed pollen extract immunotherapy
- Treatment of Patients with Refractory Atopic Dermatitis Sensitized to House Dust Mites by Using Sublingual Allergen Immunotherapy
- Two Cases of Atopic Dermatitis Improved by Combination Treatment of Allergen-Specific Immunotherapy and Histamine-Immunoglobulin Complex