Tuberc Respir Dis.
2016 Oct;79(4):257-266. 10.4046/trd.2016.79.4.257.
Increased Cellular NAD⺠Level through NQO1 Enzymatic Action Has Protective Effects on Bleomycin-Induced Lung Fibrosis in Mice
- Affiliations
-
- 1Department of Microbiology, Center for Metabolic Function Regulation, Wonkwang University School of Medicine, Iksan, Korea. jeanso@wku.ac.kr
- 2Department of Internal Medicine, Wonkwang University School of Medicine, Iksan, Korea. yshpul@wku.ac.kr
- KMID: 2365317
- DOI: http://doi.org/10.4046/trd.2016.79.4.257
Abstract
- BACKGROUND
Idiopathic pulmonary fibrosis is a common interstitial lung disease; it is a chronic, progressive, and fatal lung disease of unknown etiology. Over the last two decades, knowledge about the underlying mechanisms of pulmonary fibrosis has improved markedly and facilitated the identification of potential targets for novel therapies. However, despite the large number of antifibrotic drugs being described in experimental pre-clinical studies, the translation of these findings into clinical practices has not been accomplished yet. NADH:quinone oxidoreductase 1 (NQO1) is a homodimeric enzyme that catalyzes the oxidation of NADH to NAD+ by various quinones and thereby elevates the intracellular NAD⺠levels. In this study, we examined the effect of increase in cellular NAD⺠levels on bleomycin-induced lung fibrosis in mice.
METHODS
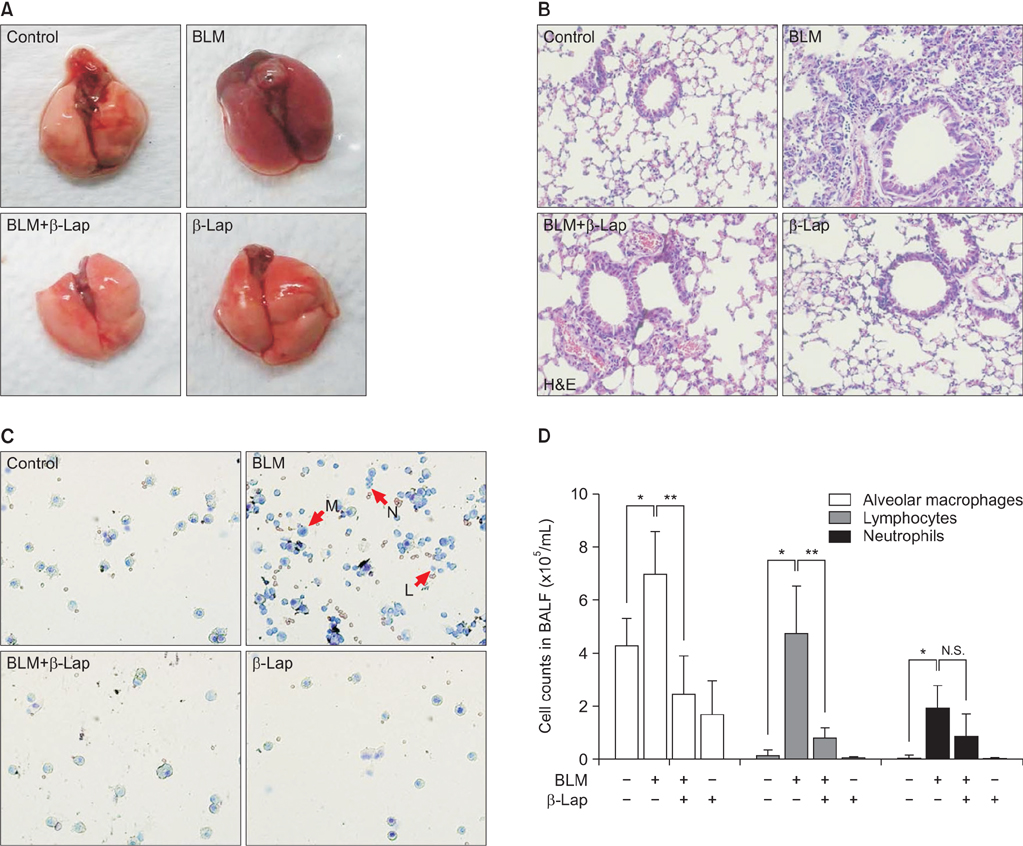
C57BL/6 mice were treated with intratracheal instillation of bleomycin. The mice were orally administered with β-lapachone from 3 days before exposure to bleomycin to 1-3 weeks after exposure to bleomycin. Bronchoalveolar lavage fluid (BALF) was collected for analyzing the infiltration of immune cells. In vitro, A549 cells were treated with transforming growth factor β1 (TGF-β1) and β-lapachone to analyze the extracellular matrix (ECM) and epithelial-mesenchymal transition (EMT).
RESULTS
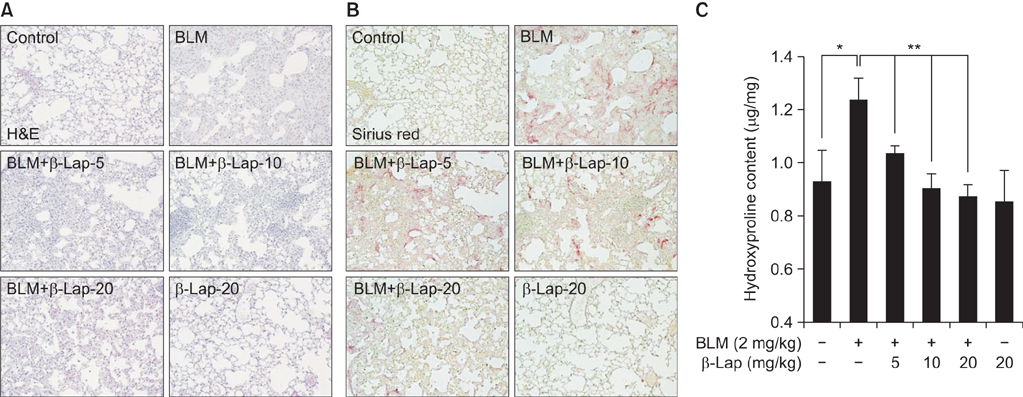
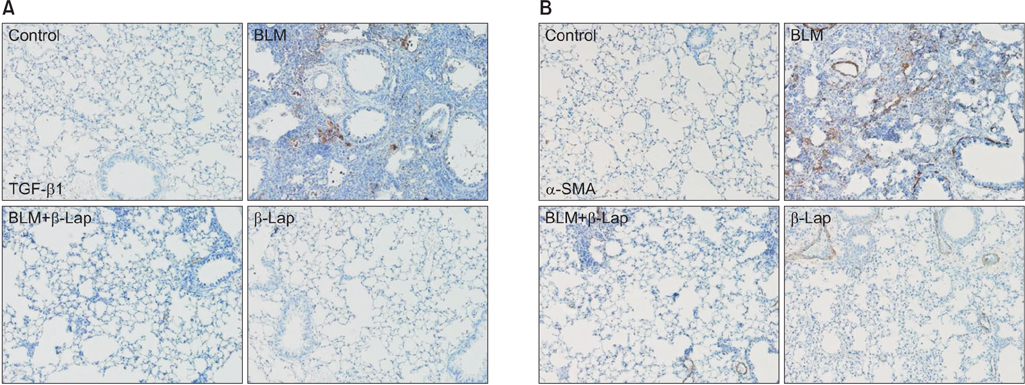
β-Lapachone strongly attenuated bleomycin-induced lung inflammation and fibrosis, characterized by histological staining, infiltrated immune cells in BALF, inflammatory cytokines, fibrotic score, and TGF-β1, α-smooth muscle actin accumulation. In addition, β-lapachone showed a protective role in TGF-β1-induced ECM expression and EMT in A549 cells.
CONCLUSION
Our results suggest that β-lapachone can protect against bleomycin-induced lung inflammation and fibrosis in mice and TGF-β1-induced EMT in vitro, by elevating the NAD+/NADH ratio through NQO1 activation.
Keyword
MeSH Terms
-
Actins
Animals
Bleomycin
Bronchoalveolar Lavage Fluid
Cytokines
Epithelial-Mesenchymal Transition
Extracellular Matrix
Fibrosis*
Idiopathic Pulmonary Fibrosis
In Vitro Techniques
Inflammation
Lung Diseases
Lung Diseases, Interstitial
Lung*
Mice*
NAD
Pneumonia
Pulmonary Fibrosis
Quinones
Transforming Growth Factor beta1
Transforming Growth Factors
Actins
Bleomycin
Cytokines
NAD
Quinones
Transforming Growth Factor beta1
Transforming Growth Factors
Figure
Reference
-
1. Spagnolo P, Sverzellati N, Rossi G, Cavazza A, Tzouvelekis A, Crestani B, et al. Idiopathic pulmonary fibrosis: an update. Ann Med. 2015; 47:15–27.2. Prasad R, Gupta N, Singh A, Gupta P. Diagnosis of idiopathic pulmonary fibrosis: current issues. Intractable Rare Dis Res. 2015; 4:65–69.3. Woodcock HV, Maher TM. The treatment of idiopathic pulmonary fibrosis. F1000Prime Re. 2014; 6:16.4. Todd NW, Luzina IG, Atamas SP. Molecular and cellular mechanisms of pulmonary fibrosis. Fibrogenesis Tissue Repair. 2012; 5:11.5. Jenkins G, Goodwin A. Novel approaches to pulmonary fibrosis. Clin Med (Lond). 2014; 14:Suppl 6. S45–S49.6. Moeller A, Ask K, Warburton D, Gauldie J, Kolb M. The bleomycin animal model: a useful tool to investigate treatment options for idiopathic pulmonary fibrosis? Int J Biochem Cell Biol. 2008; 40:362–382.7. American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS). Am J Respir Crit Care Med. 2000; 161(2 Pt 1):646–664.8. Troy L, Corte T. Interstitial lung disease in 2015: where are we now? Aust Fam Physician. 2015; 44:546–552.9. Williamson JD, Sadofsky LR, Hart SP. The pathogenesis of bleomycin-induced lung injury in animals and its applicability to human idiopathic pulmonary fibrosis. Exp Lung Res. 2015; 41:57–73.10. Impellizzeri D, Talero E, Siracusa R, Alcaide A, Cordaro M, Maria Zubelia J, et al. Protective effect of polyphenols in an inflammatory process associated with experimental pulmonary fibrosis in mice. Br J Nutr. 2015; 114:853–865.11. Chaudhary NI, Schnapp A, Park JE. Pharmacologic differentiation of inflammation and fibrosis in the rat bleomycin model. Am J Respir Crit Care Med. 2006; 173:769–776.12. Phan SH, Kunkel SL. Lung cytokine production in bleomycin-induced pulmonary fibrosis. Exp Lung Res. 1992; 18:29–43.13. Houtkooper RH, Canto C, Wanders RJ, Auwerx J. The secret life of NAD+: an old metabolite controlling new metabolic signaling pathways. Endocr Rev. 2010; 31:194–223.14. Ido Y. Pyridine nucleotide redox abnormalities in diabetes. Antioxid Redox Signal. 2007; 9:931–942.15. Ying W. NAD+/NADH and NADP+/NADPH in cellular functions and cell death: regulation and biological consequences. Antioxid Redox Signal. 2008; 10:179–206.16. Braidy N, Guillemin GJ, Mansour H, Chan-Ling T, Poljak A, Grant R. Age related changes in NAD+ metabolism oxidative stress and Sirt1 activity in wistar rats. PLoS One. 2011; 6:e19194.17. Massudi H, Grant R, Braidy N, Guest J, Farnsworth B, Guillemin GJ. Age-associated changes in oxidative stress and NAD+ metabolism in human tissue. PLoS One. 2012; 7:e42357.18. Kim HJ, Oh GS, Shen A, Lee SB, Choe SK, Kwon KB, et al. Augmentation of NAD(+) by NQO1 attenuates cisplatin-mediated hearing impairment. Cell Death Dis. 2014; 5:e1292.19. Oh GS, Kim HJ, Choi JH, Shen A, Choe SK, Karna A, et al. Pharmacological activation of NQO1 increases NAD(+) levels and attenuates cisplatin-mediated acute kidney injury in mice. Kidney Int. 2014; 85:547–560.20. Ross D, Kepa JK, Winski SL, Beall HD, Anwar A, Siegel D. NAD(P)H:quinone oxidoreductase 1 (NQO1): chemoprotection, bioactivation, gene regulation and genetic polymorphisms. Chem Biol Interact. 2000; 129:77–97.21. Gaikwad A, Long DJ 2nd, Stringer JL, Jaiswal AK. In vivo role of NAD(P)H:quinone oxidoreductase 1 (NQO1) in the regulation of intracellular redox state and accumulation of abdominal adipose tissue. J Biol Chem. 2001; 276:22559–22564.22. Pardee AB, Li YZ, Li CJ. Cancer therapy with beta-lapachone. Curr Cancer Drug Targets. 2002; 2:227–242.23. Li LS, Bey EA, Dong Y, Meng J, Patra B, Yan J, et al. Modulating endogenous NQO1 levels identifies key regulatory mechanisms of action of beta-lapachone for pancreatic cancer therapy. Clin Cancer Res. 2011; 17:275–285.24. Planchon SM, Wuerzberger S, Frydman B, Witiak DT, Hutson P, Church DR, et al. Beta-lapachone-mediated apoptosis in human promyelocytic leukemia (HL-60) and human prostate cancer cells: a p53-independent response. Cancer Res. 1995; 55:3706–3711.25. Hwang JH, Kim DW, Jo EJ, Kim YK, Jo YS, Park JH, et al. Pharmacological stimulation of NADH oxidation ameliorates obesity and related phenotypes in mice. Diabetes. 2009; 58:965–974.26. Kim SY, Jeoung NH, Oh CJ, Choi YK, Lee HJ, Kim HJ, et al. Activation of NAD(P)H:quinone oxidoreductase 1 prevents arterial restenosis by suppressing vascular smooth muscle cell proliferation. Circ Res. 2009; 104:842–850.27. Kim YH, Hwang JH, Noh JR, Gang GT, Kim DH, Son HY, et al. Activation of NAD(P)H:quinone oxidoreductase ameliorates spontaneous hypertension in an animal model via modulation of eNOS activity. Cardiovasc Res. 2011; 91:519–527.28. Lee JS, Park AH, Lee SH, Kim JH, Yang SJ, Yeom YI, et al. Beta-lapachone, a modulator of NAD metabolism, prevents health declines in aged mice. PLoS One. 2012; 7:e47122.29. Gustafson DL, Siegel D, Rastatter JC, Merz AL, Parpal JC, Kepa JK, et al. Kinetics of NAD(P)H:quinone oxidoreductase I (NQO1) inhibition by mitomycin C in vitro and in vivo. J Pharmacol Exp Ther. 2003; 305:1079–1086.30. Green FH. Overview of pulmonary fibrosis. Chest. 2002; 122:6 Suppl. 334S–339S.31. Kendall RT, Feghali-Bostwick CA. Fibroblasts in fibrosis: novel roles and mediators. Front Pharmacol. 2014; 5:123.32. Coward WR, Saini G, Jenkins G. The pathogenesis of idiopathic pulmonary fibrosis. Ther Adv Respir Dis. 2010; 4:367–388.33. Kage H, Borok Z. EMT and interstitial lung disease: a mysterious relationship. Curr Opin Pulm Med. 2012; 18:517–523.34. Willis BC, Borok Z. TGF-beta-induced EMT: mechanisms and implications for fibrotic lung disease. Am J Physiol Lung Cell Mol Physiol. 2007; 293:L525–L534.35. Idiopathic Pulmonary Fibrosis Clinical Research Network. Raghu G, Anstrom KJ, King TE Jr, Lasky JA, Martinez FJ. Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis. N Engl J Med. 2012; 366:1968–1977.36. Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014; 370:2071–2082.37. King TE Jr, Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014; 370:2083–2092.38. Taniguchi H, Ebina M, Kondoh Y, Ogura T, Azuma A, Suga M, et al. Pirfenidone in idiopathic pulmonary fibrosis. Eur Respir J. 2010; 35:821–829.39. Zhu H, Li Y. NAD(P)H: quinone oxidoreductase 1 and its potential protective role in cardiovascular diseases and related conditions. Cardiovasc Toxicol. 2012; 12:39–45.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Deficiency of Sphingosine-1-Phosphate Receptor 2 (S1Pâ‚‚) Attenuates Bleomycin-Induced Pulmonary Fibrosis
- Effects of aging on accompanying intermittent hypoxia in a bleomycin-induced pulmonary fibrosis mouse model
- Early and Late Changes of MMP-2 and MMP-9 in Bleomycin-Induced Pulmonary Fibrosis
- Utility of Micro CT in a Murine Model of Bleomycin-Induced Lung Fibrosis
- Experimental Study of the Pulmonary Toxicity of Combined Bleomycin and Captopril Administration in Mice