Korean J Physiol Pharmacol.
2016 Nov;20(6):573-580. 10.4196/kjpp.2016.20.6.573.
Novel functional roles of caspase-related genes in the regulation of apoptosis and autophagy
- Affiliations
-
- 1Biomedical Research Center, KAIST, Daejeon 34141, Korea.
- 2New Drug Development Center, DGMIF, Daegu 41061, Korea. shmin03@dgmif.re.kr
- 3LegoChem Biosciences, Inc., Daejeon 34302, Korea.
- KMID: 2364261
- DOI: http://doi.org/10.4196/kjpp.2016.20.6.573
Abstract
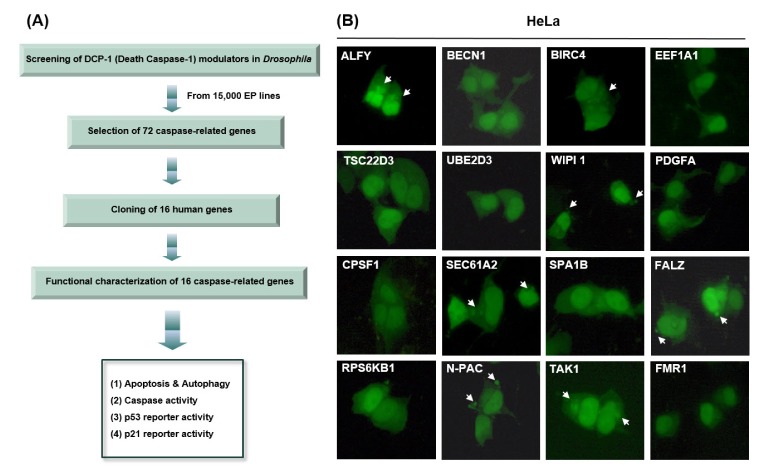
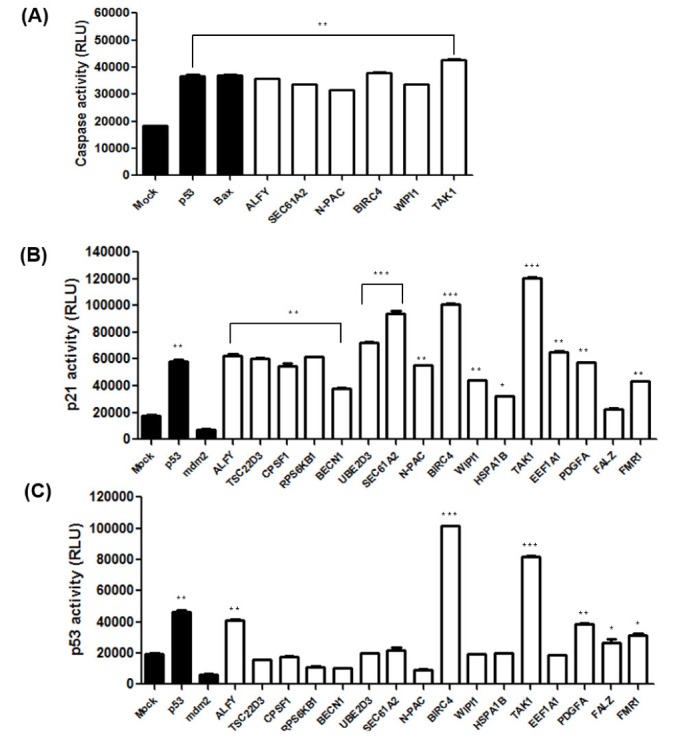
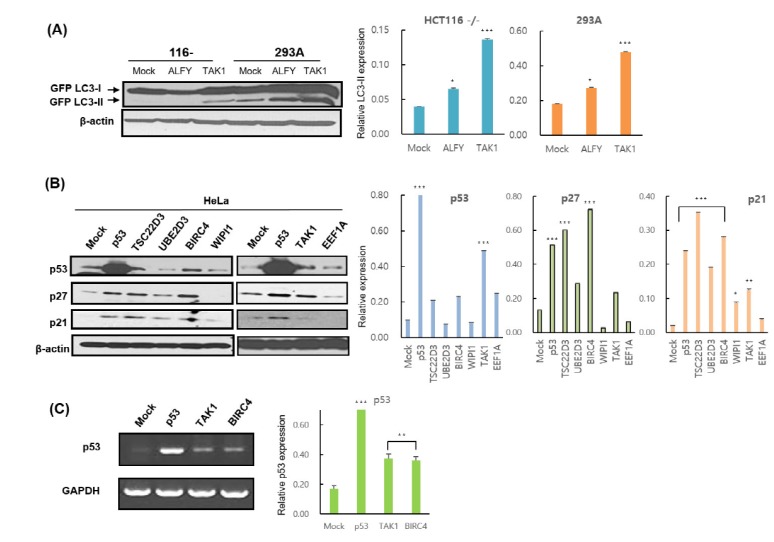
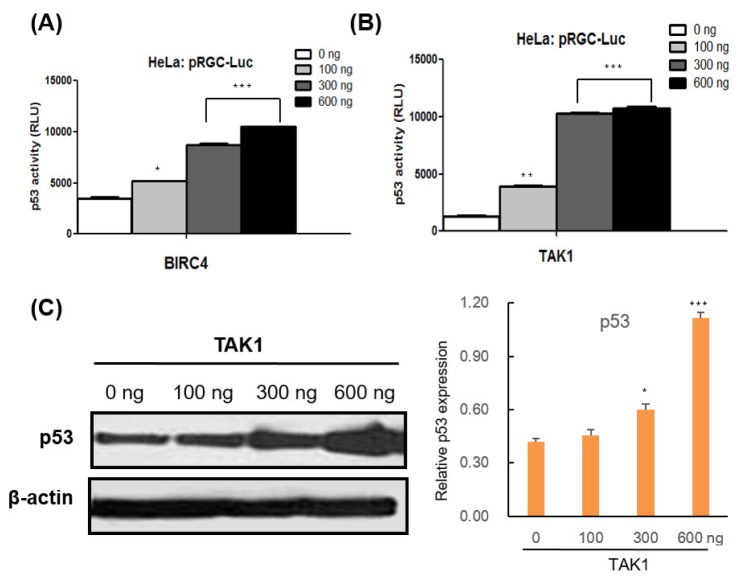
- Caspases, a family of cysteine proteases, cleave substrates and play significant roles in apoptosis, autophagy, and development. Recently, our group identified 72 genes that interact with Death Caspase-1 (DCP-1) proteins in Drosophila by genetic screening of 15,000 EP lines. However, the cellular functions and molecular mechanisms of the screened genes, such as their involvement in apoptosis and autophagy, are poorly understood in mammalian cells. In order to study the functional characterizations of the genes in human cells, we investigated 16 full-length human genes in mammalian expression vectors and tested their effects on apoptosis and autophagy in human cell lines. Our studies revealed that ALFY, BIRC4, and TAK1 induced autophagy, while SEC61A2, N-PAC, BIRC4, WIPI1, and FALZ increased apoptotic cell death. BIRC4 was involved in both autophagy and apoptosis. Western blot analysis and luciferase reporter activity indicated that ALFY, BIRC4, PDGFA, and TAK1 act in a p53-dependent manner, whereas CPSF1, SEC61A2, N-PAC, and WIPI1 appear to be p53-independent. Overexpression of BIRC4 and TAK1 caused upregulation of p53 and accumulation of its target proteins as well as an increase in p53 mRNA levels, suggesting that these genes are involved in p53 transcription and expression of its target genes followed by p53 protein accumulation. In conclusion, apoptosis and/or autophagy mediated by BIRC4 and TAK1 may be regulated by p53 and caspase activity. These novel findings may provide valuable information that will aid in a better understanding of the roles of caspase-related genes in human cell lines and be useful for the process of drug discovery.
MeSH Terms
Figure
Reference
-
1. Kim YI, Ryu T, Lee J, Heo YS, Ahnn J, Lee SJ, Yoo O. A genetic screen for modifiers of Drosophila caspase Dcp-1 reveals caspase involvement in autophagy and novel caspase-related genes. BMC Cell Biol. 2010; 11:9. PMID: 20100334.
Article2. Klionsky DJ, Abeliovich H, Agostinis P, Agrawal DK, Aliev G, Askew DS, Baba M, Baehrecke EH, Bahr BA, Ballabio A, Bamber BA, Bassham DC, Bergamini E, Bi X, Biard-Piechaczyk M, Blum JS, Bredesen DE, Brodsky JL, Brumell JH, Brunk UT, Bursch W, Camougrand N, Cebollero E, Cecconi F, Chen Y, Chin LS, Choi A, Chu CT, Chung J, Clarke PG, Clark RS, Clarke SG, Clavé C, Cleveland JL, Codogno P, Colombo MI, Coto-Montes A, Cregg JM, Cuervo AM, Debnath J, Demarchi F, Dennis PB, Dennis PA, Deretic V, Devenish RJ, Di Sano F, Dice JF, Difiglia M, Dinesh-Kumar S, Distelhorst CW, Djavaheri-Mergny M, Dorsey FC, Dröge W, Dron M, Dunn WA Jr, Duszenko M, Eissa NT, Elazar Z, Esclatine A, Eskelinen EL, Fésüs L, Finley KD, Fuentes JM, Fueyo J, Fujisaki K, Galliot B, Gao FB, Gewirtz DA, Gibson SB, Gohla A, Goldberg AL, Gonzalez R, González-Estévez C, Gorski S, Gottlieb RA, Häussinger D, He YW, Heidenreich K, Hill JA, Høyer-Hansen M, Hu X, Huang WP, Iwasaki A, Jäättelä M, Jackson WT, Jiang X, Jin S, Johansen T, Jung JU, Kadowaki M, Kang C, Kelekar A, Kessel DH, Kiel JA, Kim HP, Kimchi A, Kinsella TJ, Kiselyov K, Kitamoto K, Knecht E, Komatsu M, Kominami E, Kondo S, Kovács AL, Kroemer G, Kuan CY, Kumar R, Kundu M, Landry J, Laporte M, Le W, Lei HY, Lenardo MJ, Levine B, Lieberman A, Lim KL, Lin FC, Liou W, Liu LF, Lopez-Berestein G, López-Otín C, Lu B, Macleod KF, Malorni W, Martinet W, Matsuoka K, Mautner J, Meijer AJ, Meléndez A, Michels P, Miotto G, Mistiaen WP, Mizushima N, Mograbi B, Monastyrska I, Moore MN, Moreira PI, Moriyasu Y, Motyl T, Münz C, Murphy LO, Naqvi NI, Neufeld TP, Nishino I, Nixon RA, Noda T, Nürnberg B, Ogawa M, Oleinick NL, Olsen LJ, Ozpolat B, Paglin S, Palmer GE, Papassideri I, Parkes M, Perlmutter DH, Perry G, Piacentini M, Pinkas-Kramarski R, Prescott M, Proikas-Cezanne T, Raben N, Rami A, Reggiori F, Rohrer B, Rubinsztein DC, Ryan KM, Sadoshima J, Sakagami H, Sakai Y, Sandri M, Sasakawa C, Sass M, Schneider C, Seglen PO, Seleverstov O, Settleman J, Shacka JJ, Shapiro IM, Sibirny A, Silva-Zacarin EC, Simon HU, Simone C, Simonsen A, Smith MA, Spanel-Borowski K, Srinivas V, Steeves M, Stenmark H, Stromhaug PE, Subauste CS, Sugimoto S, Sulzer D, Suzuki T, Swanson MS, Tabas I, Takeshita F, Talbot NJ, Tallóczy Z, Tanaka K, Tanaka K, Tanida I, Taylor GS, Taylor JP, Terman A, Tettamanti G, Thompson CB, Thumm M, Tolkovsky AM, Tooze SA, Truant R, Tumanovska LV, Uchiyama Y, Ueno T, Uzcátegui NL, van der Klei I, Vaquero EC, Vellai T, Vogel MW, Wang HG, Webster P, Wiley JW, Xi Z, Xiao G, Yahalom J, Yang JM, Yap G, Yin XM, Yoshimori T, Yu L, Yue Z, Yuzaki M, Zabirnyk O, Zheng X, Zhu X, Deter RL. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy. 2008; 4:151–175. PMID: 18188003.
Article3. Kondo Y, Kanzawa T, Sawaya R, Kondo S. The role of autophagy in cancer development and response to therapy. Nat Rev Cancer. 2005; 5:726–734. PMID: 16148885.
Article4. Meijer AJ, Codogno P. Regulation and role of autophagy in mammalian cells. Int J Biochem Cell Biol. 2004; 36:2445–2462. PMID: 15325584.
Article5. Kourtis N, Tavernarakis N. Autophagy and cell death in model organisms. Cell Death Differ. 2009; 16:21–30. PMID: 19079286.
Article6. Denton D, Nicolson S, Kumar S. Cell death by autophagy: facts and apparent artefacts. Cell Death Differ. 2012; 19:87–95. PMID: 22052193.
Article7. Adhikari A, Xu M, Chen ZJ. Ubiquitin-mediated activation of TAK1 and IKK. Oncogene. 2007; 26:3214–3226. PMID: 17496917.
Article8. Ruvinsky I, Meyuhas O. Ribosomal protein S6 phosphorylation: from protein synthesis to cell size. Trends Biochem Sci. 2006; 31:342–348. PMID: 16679021.
Article9. Liu JL, Mao Z, Gallick GE, Yung WK. AMPK/TSC2/mTOR-signaling intermediates are not necessary for LKB1-mediated nuclear retention of PTEN tumor suppressor. Neuro Oncol. 2011; 13:184–194. PMID: 21123367.
Article10. Scarlatti F, Granata R, Meijer AJ, Codogno P. Does autophagy have a license to kill mammalian cells? Cell Death Differ. 2009; 16:12–20. PMID: 18600232.
Article11. Landström M. The TAK1-TRAF6 signalling pathway. Int J Biochem Cell Biol. 2010; 42:585–589. PMID: 20060931.
Article12. Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell. 2010; 140:313–326. PMID: 20144757.
Article13. Maiuri MC, Tasdemir E, Criollo A, Morselli E, Vicencio JM, Carnuccio R, Kroemer G. Control of autophagy by oncogenes and tumor suppressor genes. Cell Death Differ. 2009; 16:87–93. PMID: 18806760.
Article14. Castedo M, Ferri KF, Kroemer G. Mammalian target of rapamycin (mTOR): pro- and anti-apoptotic. Cell Death Differ. 2002; 9:99–100. PMID: 11840159.
Article15. Xie M, Zhang D, Dyck JR, Li Y, Zhang H, Morishima M, Mann DL, Taffet GE, Baldini A, Khoury DS, Schneider MD. A pivotal role for endogenous TGF-beta-activated kinase-1 in the LKB1/AMP-activated protein kinase energy-sensor pathway. Proc Natl Acad Sci U S A. 2006; 103:17378–17383. PMID: 17085580.
Article16. Levine B. Cell biology: autophagy and cancer. Nature. 2007; 446:745–747. PMID: 17429391.17. Jin S. p53, Autophagy and tumor suppression. Autophagy. 2005; 1:171–173. PMID: 16874039.
Article18. Crighton D, Wilkinson S, Ryan KM. DRAM links autophagy to p53 and programmed cell death. Autophagy. 2007; 3:72–74. PMID: 17102582.
Article19. Tasdemir E, Maiuri MC, Galluzzi L, Vitale I, Djavaheri-Mergny M, D'Amelio M, Criollo A, Morselli E, Zhu C, Harper F, Nannmark U, Samara C, Pinton P, Vicencio JM, Carnuccio R, Moll UM, Madeo F, Paterlini-Brechot P, Rizzuto R, Szabadkai G, Pierron G, Blomgren K, Tavernarakis N, Codogno P, Cecconi F, Kroemer G. Regulation of autophagy by cytoplasmic p53. Nat Cell Biol. 2008; 10:676–687. PMID: 18454141.
Article20. Tasdemir E, Maiuri MC, Orhon I, Kepp O, Morselli E, Criollo A, Kroemer G. p53 represses autophagy in a cell cycle-dependent fashion. Cell Cycle. 2008; 7:3006–3011. PMID: 18838865.
Article21. Morselli E, Tasdemir E, Maiuri MC, Galluzzi L, Kepp O, Criollo A, Vicencio JM, Soussi T, Kroemer G. Mutant p53 protein localized in the cytoplasm inhibits autophagy. Cell Cycle. 2008; 7:3056–3061. PMID: 18818522.
Article22. Dann SG, Selvaraj A, Thomas G. mTOR Complex1-S6K1 signaling: at the crossroads of obesity, diabetes and cancer. Trends Mol Med. 2007; 13:252–259. PMID: 17452018.
Article23. Efeyan A, Sabatini DM. mTOR and cancer: many loops in one pathway. Curr Opin Cell Biol. 2010; 22:169–176. PMID: 19945836.
Article24. Jung CH, Ro SH, Cao J, Otto NM, Kim DH. mTOR regulation of autophagy. FEBS Lett. 2010; 584:1287–1295. PMID: 20083114.
Article25. Crighton D, Wilkinson S, O'Prey J, Syed N, Smith P, Harrison PR, Gasco M, Garrone O, Crook T, Ryan KM. DRAM, a p53-induced modulator of autophagy, is critical for apoptosis. Cell. 2006; 126:121–134. PMID: 16839881.
Article26. Min SH, Kim DM, Heo YS, Kim YI, Kim HM, Kim J, Han YM, Kim IC, Yoo OJ. New p53 target, phosphatase of regenerating liver 1 (PRL-1) downregulates p53. Oncogene. 2009; 28:545–554. PMID: 18997816.
Article27. Min SH, Lau AW, Lee TH, Inuzuka H, Wei S, Huang P, Shaik S, Lee DY, Finn G, Balastik M, Chen CH, Luo M, Tron AE, Decaprio JA, Zhou XZ, Wei W, Lu KP. Negative regulation of the stability and tumor suppressor function of Fbw7 by the Pin1 prolyl isomerase. Mol Cell. 2012; 46:771–783. PMID: 22608923.
Article28. Shin JH, Min SH, Kim SJ, Kim YI, Park J, Lee HK, Yoo OJ. TAK1 regulates autophagic cell death by suppressing the phosphorylation of p70 S6 kinase 1. Sci Rep. 2013; 3:1561. PMID: 23532117.
Article29. Crighton D, Wilkinson S, O'Prey J, Syed N, Smith P, Harrison PR, Gasco M, Garrone O, Crook T, Ryan KM. DRAM, a p53-induced modulator of autophagy, is critical for apoptosis. Cell. 2006; 126:121–134. PMID: 16839881.
Article30. Yang JY, Park MY, Park SY, Yoo HI, Kim MS, Kim JH, Kim WJ, Jung JY. Nitric Oxide-induced autophagy in MC3T3-E1 cells is associated with cytoprotection via AMPK activation. Korean J Physiol Pharmacol. 2015; 19:507–514. PMID: 26557017.
Article31. Kim SI, Lee WK, Kang SS, Lee SY, Jeong MJ, Lee HJ, Kim SS, Johnson GV, Chun W. Suppression of autophagy and activation of glycogen synthase kinase 3beta facilitate the aggregate formation of tau. Korean J Physiol Pharmacol. 2011; 15:107–114. PMID: 21660151.
Article32. Ahn JH, Kim MH, Kwon HJ, Choi SY, Kwon HY. Protective effects of oleic acid against palmitic acid-induced apoptosis in pancreatic AR42J cells and its mechanisms. Korean J Physiol Pharmacol. 2013; 17:43–50. PMID: 23440052.
Article33. Yang JY, Park MY, Park SY, Yoo HI, Kim MS, Kim JH, Kim WJ, Jung JY. Nitric Oxide-induced autophagy in MC3T3-E1 cells is associated with cytoprotection via AMPK activation. Korean J Physiol Pharmacol. 2015; 19:507–514. PMID: 26557017.
Article34. Bae YH, Ryu JH, Park HJ, Kim KR, Wee HJ, Lee OH, Jang HO, Bae MK, Kim KW, Bae SK. Mutant p53-notch1 signaling axis is involved in curcumin-induced apoptosis of breast cancer cells. Korean J Physiol Pharmacol. 2013; 17:291–297. PMID: 23946688.
Article35. Connerly PL, Esaki M, Montegna EA, Strongin DE, Levi S, Soderholm J, Glick BS. Sec16 is a determinant of transitional ER organization. Curr Biol. 2005; 15:1439–1447. PMID: 16111939.
Article36. Richart L, Carrillo-de Santa Pau E, Río-Machín A, de Andrés MP, Cigudosa JC, Lobo VJ, Real FX. BPTF is required for c-MYC transcriptional activity and in vivo tumorigenesis. Nat Commun. 2016; 7:10153. PMID: 26729287.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Induction of Apoptosis and Autophagy in UVB-Treated HaCaT Cells
- Regulatory Role of Autophagy in Globular Adiponectin-Induced Apoptosis in Cancer Cells
- Dual Roles of Autophagy and Their Potential Drugs for Improving Cancer Therapeutics
- The Impact of Autophagy on the Cigarette Smoke Extract-Induced Apoptosis of Bronchial Epithelial Cells
- Nrf2-Heme oxygenase-1 modulates autophagy and inhibits apoptosis triggered by elevated glucose levels in renal tubule cells