J Korean Med Sci.
2017 Feb;32(2):329-334. 10.3346/jkms.2017.32.2.329.
Efficacy and Tolerability of Solifenacin 5 mg Fixed Dose in Korean Children with Newly Diagnosed Idiopathic Overactive Bladder: a Multicenter Prospective Study
- Affiliations
-
- 1Department of Urology, Pusan National University Yangsan Hospital and Research Institute for Convergence of Biomedical Science and Technology, Yangsan, Korea.
- 2Department of Urology, Kosin University College of Medicine, Busan, Korea.
- 3Department of Urology, Inje University Busan Paik Hospital, Busan, Korea.
- 4Department of Urology, Samsung Changwon Hospital, Sungkyunkwan University School of Medicine, Changwon, Korea.
- 5Department of Urology, Dong-A University Hospital, Dong-A University, Busan, Korea.
- 6Department of Urology, Ulsan University Hospital, University of Ulsan College of Medicine, Ulsan, Korea. scpark@amc.seoul.kr
- 7School of Life Sciences, Ulsan National Institute of Science and Technology, Ulsan, Korea.
- KMID: 2364179
- DOI: http://doi.org/10.3346/jkms.2017.32.2.329
Abstract
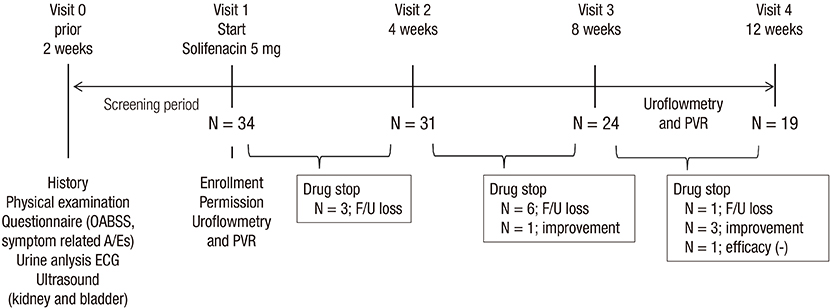
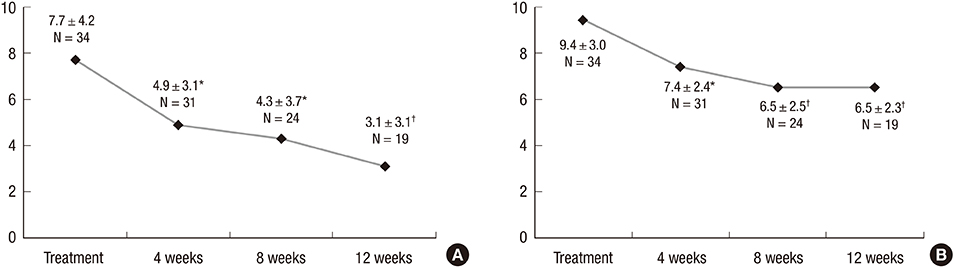
- We investigated the efficacy and tolerability of solifenacin 5 mg fixed dose in children with newly diagnosed idiopathic overactive bladder (OAB). A total of 34 children (male/female patients = 16/18) aged under 13 years (mean age: 7.2 ± 2.3; range: 5-12) who were newly diagnosed with OAB from January 2012 to September 2014 were prospectively evaluated with open-label protocol. All patients were treated with solifenacin 5 mg fixed dose once daily for at least 4 weeks. The efficacy and tolerability of solifenacin were evaluated 4, 8, and 12 weeks after the initiation of treatment. The mean voiding frequency during daytime was decreased from 9.4 ± 3.0 to 6.5 ± 2.3 times after the 12-week treatment (P < 0.001). The mean total OAB symptom score (OABSS) decreased from 7.7 ± 4.2 to 3.1 ± 3.1 after the 12-week treatment (P < 0.001). The urgency and urgency urinary incontinence (UUI) domains significantly improved from the 12-week treatment, and complete resolution of urgency occurred in 38.9% of patients and the percentage of children with UUI among urgent patients decreased from 79.4% to 57.1%. According to 3-day voiding diaries, the average bladder capacity increased from 90.4 ± 44.4 to 156.2 ± 67.3 mL (P < 0.001). Drug-induced adverse effects (AEs) were reported in 7 patients (20.6%). Our results indicate that solifenacin 5 mg fixed dose is effective against OAB symptoms, and its tolerability is acceptable without significant AEs in children with OAB.
Keyword
MeSH Terms
Figure
Reference
-
1. Schröder A, Thüroff JW. New strategies for medical management of overactive bladder in children. Curr Opin Urol. 2010; 20:313–317.2. Marschall-Kehrel D, Feustel C, Persson de Geeter C, Stehr M, Radmayr C, Sillén U, Strugala G. Treatment with propiverine in children suffering from nonneurogenic overactive bladder and urinary incontinence: results of a randomized placebo-controlled phase 3 clinical trial. Eur Urol. 2009; 55:729–736.3. Schulte-Baukloh H, Mürtz G, Heine G, Austin P, Miller K, Michael T, Strugala G, Knispel HH. Urodynamic effects of propiverine in children and adolescents with neurogenic bladder: results of a prospective long-term study. J Pediatr Urol. 2012; 8:386–392.4. Bolduc S, Moore K, Lebel S, Lamontagne P, Hamel M. Double anticholinergic therapy for refractory overactive bladder. J Urol. 2009; 182:2033–2038.5. Bolduc S, Moore K, Nadeau G, Lebel S, Lamontagne P, Hamel M. Prospective open label study of solifenacin for overactive bladder in children. J Urol. 2010; 184:1668–1673.6. Chapple CR, Rechberger T, Al-Shukri S, Meffan P, Everaert K, Huang M, Ridder A. YM-905 Study Group. Randomized, double-blind placebo- and tolterodine-controlled trial of the once-daily antimuscarinic agent solifenacin in patients with symptomatic overactive bladder. BJU Int. 2004; 93:303–310.7. Hoebeke P, De Pooter J, De Caestecker K, Raes A, Dehoorne J, Van Laecke E, Vande Walle J. Solifenacin for therapy resistant overactive bladder. J Urol. 2009; 182:2040–2044.8. Yamaguchi O, Kakizaki H, Homma Y, Igawa Y, Takeda M, Nishizawa O, Gotoh M, Yoshida M, Yokoyama O, Seki N, et al. Safety and efficacy of mirabegron as ‘add-on’ therapy in patients with overactive bladder treated with solifenacin: a post-marketing, open-label study in Japan (MILAI study). BJU Int. 2015; 116:612–622.9. Jeong SJ, Homma Y, Oh SJ. Reproducibility study of overactive bladder symptom score questionnaire and its response to treatment (RESORT) in Korean population with overactive bladder symptoms. Qual Life Res. 2014; 23:285–292.10. Lencioni A, Hutchins L, Annis S, Chen W, Ermisoglu E, Feng Z, Mack K, Simpson K, Lane C, Topaloglu U. An adverse event capture and management system for cancer studies. BMC Bioinformatics. 2015; 16:Suppl 13. S6.11. Cardozo L, Thorpe A, Warner J, Sidhu M. The cost-effectiveness of solifenacin vs fesoterodine, oxybutynin immediate-release, propiverine, tolterodine extended-release and tolterodine immediate-release in the treatment of patients with overactive bladder in the UK National Health Service. BJU Int. 2010; 106:506–514.12. Chung JM, Lee SD, Kang DI, Kwon DD, Kim KS, Kim SY, Kim HG, Moon DG, Park KH, Park YH, et al. Prevalence and associated factors of overactive bladder in Korean children 5-13 years old: a nationwide multicenter study. Urology. 2009; 73:63–67.13. Park SJ, Pai KS, Kim JM, Park K, Kim KS, Song SH, Park S, Kim SO, Ryu DS, Baek M, et al. Efficacy and tolerability of anticholinergics in Korean children with overactive bladder: a multicenter retrospective study. J Korean Med Sci. 2014; 29:1550–1554.14. Alloussi S, Mürtz G, Braun R, Gerhardt U, Heinrich M, Hellmis E, Horn W, Marschall-Kehrel D, Niklas K, Raabe M, et al. Efficacy, tolerability and safety of propiverine hydrochloride in comparison to oxybutynin in children with urge incontinence due to overactive bladder: results of a multicentre observational cohort study. BJU Int. 2010; 106:550–556.15. McKeage K. Propiverine: a review of its use in the treatment of adults and children with overactive bladder associated with idiopathic or neurogenic detrusor overactivity, and in men with lower urinary tract symptoms. Clin Drug Investig. 2013; 33:71–91.16. Malhotra B, El-Tahtawy A, Wang EQ, Darekar A, Cossons N, Crook TJ, Scholfield D, Reddy P. Dose-escalating study of the pharmacokinetics and tolerability of fesoterodine in children with overactive bladder. J Pediatr Urol. 2012; 8:336–342.17. Kim SO, Kim KD, Kim YS, Kim JM, Moon G, Park S, Lee SD, Chung JM, Cho WY; Korean Children’s Continence and Enuresis Society. Evaluation of maximum voided volume in Korean children by use of a 48-h frequency volume chart. BJU Int. 2012; 110:597–600.18. Wada Y, Yoshida M, Kitani K, Kikukawa H, Ichinose A, Takahashi W, Gotoh S, Inadome A, Machida J, Ueda S. Comparison of the effects of various anticholinergic drugs on human isolated urinary bladder. Arch Int Pharmacodyn Ther. 1995; 330:76–89.19. Chapple CR, Araño P, Bosch JL, De Ridder D, Kramer AE, Ridder AM. Solifenacin appears effective and well tolerated in patients with symptomatic idiopathic detrusor overactivity in a placebo- and tolterodine-controlled phase 2 dose-finding study. BJU Int. 2004; 93:71–77.20. Nitti VW, Khullar V, van Kerrebroeck P, Herschorn S, Cambronero J, Angulo JC, Blauwet MB, Dorrepaal C, Siddiqui E, Martin NE. Mirabegron for the treatment of overactive bladder: a prespecified pooled efficacy analysis and pooled safety analysis of three randomised, double-blind, placebo-controlled, phase III studies. Int J Clin Pract. 2013; 67:619–632.21. Maman K, Aballea S, Nazir J, Desroziers K, Neine ME, Siddiqui E, Odeyemi I, Hakimi Z. Comparative efficacy and safety of medical treatments for the management of overactive bladder: a systematic literature review and mixed treatment comparison. Eur Urol. 2014; 65:755–765.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Efficacy and tolerability of mirabegron compared with solifenacin for children with idiopathic overactive bladder: A preliminary study
- Efficacy and Tolerability of Solifenacin Fumarate with Overactive Bladder Patients: A Multicenter Observational Study
- Antimuscarinic Agent Treatment Affecting Patient-Reported Outcomes in Overactive Bladder Syndrome With Depressive Symptoms
- Review of the Anticholinergics for the Treatment of Overactive Bladder: 2009 Update
- Clinical Efficacy of Solifenacin in the Management of Diabetes Mellitus-Associated Versus Idiopathic Overactive Bladder Symptoms: A Multicenter Prospective Study