J Korean Med Sci.
2017 Feb;32(2):264-271. 10.3346/jkms.2017.32.2.264.
The FOXO1 Gene-Obesity Interaction Increases the Risk of Type 2 Diabetes Mellitus in a Chinese Han Population
- Affiliations
-
- 1Department of Endocrinology, the First Affiliated Hospital of Chongqing Medical University, Chongqing, China. lilin.gong@outlook.com
- 2Laboratory for Disease and Gene, Key Laboratory of Molecular Biology of Infectious Diseases designated by the Chinese Ministry of Education, Department of Public Health, the First Affiliated Hospital of Chongqing Medical University, Chongqing, China.
- 3Laboratory of Disorders Genes and Department of Pharmacology, Jishou University, Jishou, China.
- KMID: 2364170
- DOI: http://doi.org/10.3346/jkms.2017.32.2.264
Abstract
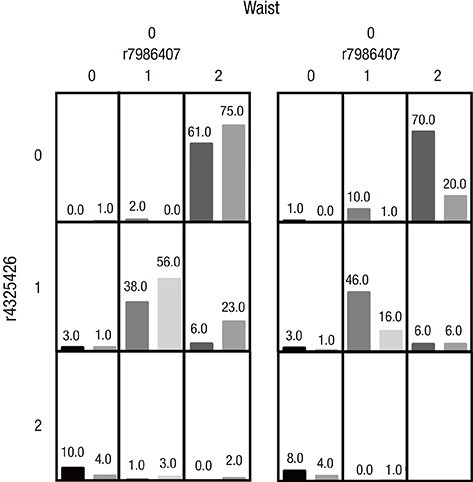
- Here, we aimed to study the effect of the forkhead box O1-insulin receptor substrate 2 (FOXO1-IRS2) gene interaction and the FOXO1 and IRS2 genes-environment interaction for the risk of type 2 diabetes mellitus (T2DM) in a Chinese Han population. We genotyped 7 polymorphism sites of FOXO1 gene and IRS2 gene in 780 unrelated Chinese Han people (474 cases of T2DM, 306 cases of healthy control). The risk of T2DM in individuals with AA genotype for rs7986407 and CC genotype for rs4581585 in FOXO1 gene was 2.092 and 2.57 times higher than that with GG genotype (odds ratio [OR] = 2.092; 95% confidence interval [CI] = 1.178-3.731; P = 0.011) and TT genotype (OR = 2.571; 95% CI = 1.404-4.695; P = 0.002), respectively. The risk of T2DM in individuals with GG genotype for Gly1057Asp in IRS2 gene was 1.42 times higher than that with AA genotype (OR = 1.422; 95% CI = 1.037-1.949; P = 0.029). The other 4 single nucleotide polymorphisms (SNPs) had no significant association with T2DM (P > 0.05). Multifactor dimensionality reduction (MDR) analysis showed that the interaction between SNPs rs7986407 and rs4325426 in FOXO1 gene and waist was the best model confirmed by interaction analysis, closely associating with T2DM. There was an increased risk for T2DM in the case of non-obesity with genotype combined AA/CC, AA/AC or AG/AA for rs7986407 and rs4325426, and obesity with genotype AA for rs7986407 or AA for rs4325426 (OR = 3.976; 95% CI = 1.156-13.675; P value from sign test [P(sign)] = 0.025; P value from permutation test [P(perm)] = 0.000-0.001). Together, this study indicates an association of FOXO1 and IRS2 gene polymorphisms with T2DM in Chinese Han population, supporting FOXO1-obesity interaction as a key factor for the risk of T2DM.
Keyword
MeSH Terms
Figure
Reference
-
1. Edelman SV. Type II diabetes mellitus. Adv Intern Med. 1998; 43:449–500.2. Voight BF, Scott LJ, Steinthorsdottir V, Morris AP, Dina C, Welch RP, Zeggini E, Huth C, Aulchenko YS, Thorleifsson G, et al. Twelve type 2 diabetes susceptibility loci identified through large-scale association analysis. Nat Genet. 2010; 42:579–589.3. O’Rahilly S, Barroso I, Wareham NJ. Genetic factors in type 2 diabetes: the end of the beginning? Science. 2005; 307:370–373.4. Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004; 27:1047–1053.5. Chan JC, Malik V, Jia W, Kadowaki T, Yajnik CS, Yoon KH, Hu FB. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA. 2009; 301:2129–2140.6. Bouret S, Levin BE, Ozanne SE. Gene-environment interactions controlling energy and glucose homeostasis and the developmental origins of obesity. Physiol Rev. 2015; 95:47–82.7. Cornelis MC, Hu FB. Gene-environment interactions in the development of type 2 diabetes: recent progress and continuing challenges. Annu Rev Nutr. 2012; 32:245–259.8. Stumvoll M, Goldstein BJ, van Haeften TW. Type 2 diabetes: principles of pathogenesis and therapy. Lancet. 2005; 365:1333–1346.9. Haeusler RA, Hartil K, Vaitheesvaran B, Arrieta-Cruz I, Knight CM, Cook JR, Kammoun HL, Febbraio MA, Gutierrez-Juarez R, Kurland IJ, et al. Integrated control of hepatic lipogenesis versus glucose production requires FoxO transcription factors. Nat Commun. 2014; 5:5190.10. Kitamura T. The role of FOXO1 in beta-cell failure and type 2 diabetes mellitus. Nat Rev Endocrinol. 2013; 9:615–623.11. Guo S. Insulin signaling, resistance, and the metabolic syndrome: insights from mouse models into disease mechanisms. J Endocrinol. 2014; 220:T1–23.12. Oliveira JM, Rebuffat SA, Gasa R, Gomis R. Targeting type 2 diabetes: lessons from a knockout model of insulin receptor substrate 2. Can J Physiol Pharmacol. 2014; 92:613–620.13. Nakae J, Biggs WH 3rd, Kitamura T, Cavenee WK, Wright CV, Arden KC, Accili D. Regulation of insulin action and pancreatic beta-cell function by mutated alleles of the gene encoding forkhead transcription factor Foxo1. Nat Genet. 2002; 32:245–253.14. Kitamura T, Nakae J, Kitamura Y, Kido Y, Biggs WH 3rd, Wright CV, White MF, Arden KC, Accili D. The forkhead transcription factor Foxo1 links insulin signaling to Pdx1 regulation of pancreatic beta cell growth. J Clin Invest. 2002; 110:1839–1847.15. Haghani K, Bakhtiyari S. The study on the relationship between IRS-1 Gly972Arg and IRS-2 Gly1057Asp polymorphisms and type 2 diabetes in the Kurdish ethnic group in West Iran. Genet Test Mol Biomarkers. 2012; 16:1270–1276.16. Okazawa K, Yoshimasa Y, Miyamoto Y, Takahashi-Yasuno A, Miyawaki T, Masuzaki H, Hayashi T, Hosoda K, Inoue G, Nakao K. The haplotypes of the IRS-2 gene affect insulin sensitivity in Japanese patients with type 2 diabetes. Diabetes Res Clin Pract. 2005; 68:39–48.17. Karim MA, Craig RL, Wang X, Hale TC, Elbein SC. Analysis of FOXO1A as a candidate gene for type 2 diabetes. Mol Genet Metab. 2006; 88:171–177.18. Müssig K, Staiger H, Machicao F, Stancáková A, Kuusisto J, Laakso M, Thamer C, Machann J, Schick F, Claussen CD, et al. Association of common genetic variation in the FOXO1 gene with beta-cell dysfunction, impaired glucose tolerance, and type 2 diabetes. J Clin Endocrinol Metab. 2009; 94:1353–1360.19. Li T, Wu X, Zhu X, Li J, Pan L, Li P, Xin Z, Liu Y. Association analyses between the genetic polymorphisms of HNF4A and FOXO1 genes and Chinese Han patients with type 2 diabetes. Mol Cell Biochem. 2011; 353:259–265.20. Jia WP, Pang C, Chen L, Bao YQ, Lu JX, Lu HJ, Tang JL, Wu YM, Zuo YH, Jiang SY, et al. Epidemiological characteristics of diabetes mellitus and impaired glucose regulation in a Chinese adult population: the Shanghai diabetes studies, a cross-sectional 3-year follow-up study in Shanghai urban communities. Diabetologia. 2007; 50:286–292.21. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985; 28:412–419.22. Lautier C, El Mkadem SA, Renard E, Brun JF, Gris JC, Bringer J, Grigorescu F. Complex haplotypes of IRS2 gene are associated with severe obesity and reveal heterogeneity in the effect of Gly1057Asp mutation. Hum Genet. 2003; 113:34–43.23. Bodhini D, Radha V, Deepa R, Ghosh S, Majumder PP, Rao MR, Mohan V. The G1057D polymorphism of IRS-2 gene and its relationship with obesity in conferring susceptibility to type 2 diabetes in Asian Indians. Int J Obes. 2007; 31:97–102.24. Villuendas G, Botella-Carretero JI, Roldán B, Sancho J, Escobar-Morreale HF, San Millán JL. Polymorphisms in the insulin receptor substrate-1 (IRS-1) gene and the insulin receptor substrate-2 (IRS-2) gene influence glucose homeostasis and body mass index in women with polycystic ovary syndrome and non-hyperandrogenic controls. Hum Reprod. 2005; 20:3184–3191.25. Murea M, Ma L, Freedman BI. Genetic and environmental factors associated with type 2 diabetes and diabetic vascular complications. Rev Diabet Stud. 2012; 9:6–22.26. Burguera B, Agusti A, Arner P, Baltasar A, Barbe F, Barcelo A, Breton I, Cabanes T, Casanueva FF, Couce ME, et al. Critical assessment of the current guidelines for the management and treatment of morbidly obese patients. J Endocrinol Invest. 2007; 30:844–852.27. Romao I, Roth J. Genetic and environmental interactions in obesity and type 2 diabetes. J Am Diet Assoc. 2008; 108:S24–8.