Korean J Ophthalmol.
2015 Dec;29(6):396-403. 10.3341/kjo.2015.29.6.396.
Effects of Vitreomacular Traction on Ranibizumab Treatment Response in Eyes with Neovascular Age-related Macular Degeneration
- Affiliations
-
- 1Department of Ophthalmology and Inha Vision Science Laboratory, Inha University School of Medicine, Incheon, Korea. drmys@inha.ac.kr
- 2Graduate School of Medical Sciences and Engineering, Korea Advanced Institute of Science and Technology, Daejeon, Korea.
- KMID: 2363847
- DOI: http://doi.org/10.3341/kjo.2015.29.6.396
Abstract
- PURPOSE
To investigate the effects of vitreomacular traction (VMT) on ranibizumab treatment response for neovascular age-related macular degeneration (AMD).
METHODS
A retrospective review of 85 eyes of 85 patients newly diagnosed with neovascular AMD was conducted. Patients were eligible if they had received more than three consecutive monthly ranibizumab (0.50 mg) treatments and ophthalmic evaluations. Patients were classified into a VMT (+) group or VMT (-) group according to optical coherence tomography imaging. Best corrected visual acuity and central retinal thickness (CRT) measurements were obtained at three and six months after initial injection.
RESULTS
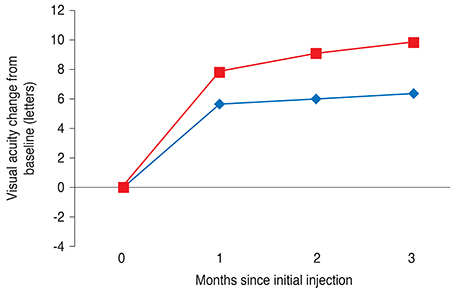
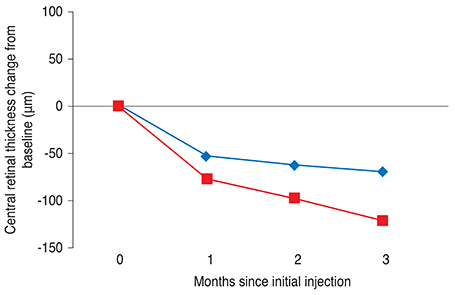
One month after the third injection, mean visual acuity (VA) increases of 6.36 and 9.87 letters were observed in the VMT (+) and VMT (-) groups, respectively. The corresponding mean CRT values decreased by 70.29 microm and 121.68 microm, respectively. A total 41 eyes were identified as eligible for a subsequent fourth injection; 71.1% of patients (27 eyes) in the VMT (+) group but only 29.8% of patients in the VMT (-) group needed a subsequent fourth injection. Follow-up was extended to six months for 42 of the 85 enrolled patients (49.4%). The trends in VA and optical coherence tomography were found to be maintained at six-month follow-up.
CONCLUSIONS
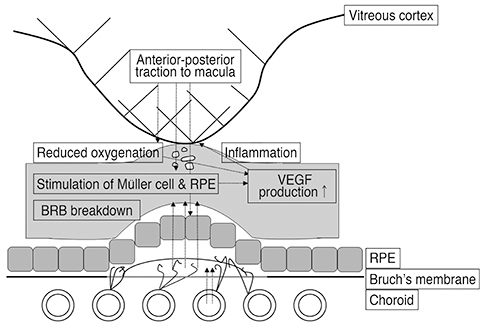
VA and CRT appeared to be more improved after ranibizumab treatment in the VMT (-) group compared to the VMT (+) group. VMT might antagonize the effect of ranibizumab treatment in a subpopulation of AMD patients.
Keyword
MeSH Terms
-
Aged
Aged, 80 and over
Angiogenesis Inhibitors/*therapeutic use
Female
Follow-Up Studies
Humans
Intravitreal Injections
Male
Middle Aged
Ranibizumab/*therapeutic use
Retina/pathology
Retinal Diseases/*physiopathology
Retrospective Studies
Tissue Adhesions
Tomography, Optical Coherence
Vascular Endothelial Growth Factor A/antagonists & inhibitors
Visual Acuity/drug effects
Vitreous Body/*pathology
Wet Macular Degeneration/*drug therapy/physiopathology
Angiogenesis Inhibitors
Ranibizumab
Vascular Endothelial Growth Factor A
Figure
Reference
-
1. Ferrara N, Damico L, Shams N, et al. Development of ranibizumab, an anti-vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina. 2006; 26:859–870.2. Ferrara N, Mass RD, Campa C, Kim R. Targeting VEGF-A to treat cancer and age-related macular degeneration. Annu Rev Med. 2007; 58:491–504.3. Witmer AN, Vrensen GF, Van Noorden CJ, Schlingemann RO. Vascular endothelial growth factors and angiogenesis in eye disease. Prog Retin Eye Res. 2003; 22:1–29.4. Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006; 355:1419–1431.5. Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006; 355:1432–1444.6. Regillo CD, Brown DM, Abraham P, et al. Randomized, double-masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER Study year 1. Am J Ophthalmol. 2008; 145:239–248.7. Schmidt-Erfurth U, Eldem B, Guymer R, et al. Efficacy and safety of monthly versus quarterly ranibizumab treatment in neovascular age-related macular degeneration: the EXCITE study. Ophthalmology. 2011; 118:831–839.8. Fung AE, Lalwani GA, Rosenfeld PJ, et al. An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol. 2007; 143:566–583.9. Holz FG, Amoaku W, Donate J, et al. Safety and efficacy of a flexible dosing regimen of ranibizumab in neovascular age-related macular degeneration: the SUSTAIN study. Ophthalmology. 2011; 118:663–671.10. CATT Research Group. Martin DF, Maguire MG, et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011; 364:1897–1908.11. Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group. Martin DF, Maguire MG, et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012; 119:1388–1398.12. IVAN Study Investigators. Chakravarthy U, Harding SP, et al. Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: one-year findings from the IVAN randomized trial. Ophthalmology. 2012; 119:1399–1411.13. Lim JH, Wickremasinghe SS, Xie J, et al. Delay to treatment and visual outcomes in patients treated with anti-vascular endothelial growth factor for age-related macular degeneration. Am J Ophthalmol. 2012; 153:678–686. 686.e1–686.e2.14. Lux A, Llacer H, Heussen FM, Joussen AM. Non-responders to bevacizumab (Avastin) therapy of choroidal neovascular lesions. Br J Ophthalmol. 2007; 91:1318–1322.15. Teper SJ, Nowinska A, Pilat J, et al. Involvement of genetic factors in the response to a variable-dosing ranibizumab treatment regimen for age-related macular degeneration. Mol Vis. 2010; 16:2598–2604.16. Klein RJ, Zeiss C, Chew EY, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005; 308:385–389.17. Dewan A, Liu M, Hartman S, et al. HTRA1 promoter polymorphism in wet age-related macular degeneration. Science. 2006; 314:989–992.18. Mori K, Horie-Inoue K, Kohda M, et al. Association of the HTRA1 gene variant with age-related macular degeneration in the Japanese population. J Hum Genet. 2007; 52:636–641.19. Krebs I, Glittenberg C, Zeiler F, Binder S. Spectral domain optical coherence tomography for higher precision in the evaluation of vitreoretinal adhesions in exudative age-related macular degeneration. Br J Ophthalmol. 2011; 95:1415–1418.20. Ondes F, Yilmaz G, Acar MA, et al. Role of the vitreous in age-related macular degeneration. Jpn J Ophthalmol. 2000; 44:91–93.21. Krebs I, Brannath W, Glittenberg C, et al. Posterior vitreomacular adhesion: a potential risk factor for exudative age-related macular degeneration? Am J Ophthalmol. 2007; 144:741–746.22. Schulze S, Hoerle S, Mennel S, Kroll P. Vitreomacular traction and exudative age-related macular degeneration. Acta Ophthalmol. 2008; 86:470–481.23. Mojana F, Cheng L, Bartsch DU, et al. The role of abnormal vitreomacular adhesion in age-related macular degeneration: spectral optical coherence tomography and surgical results. Am J Ophthalmol. 2008; 146:218–227.24. Lee SJ, Lee CS, Koh HJ. Posterior vitreomacular adhesion and risk of exudative age-related macular degeneration: paired eye study. Am J Ophthalmol. 2009; 147:621–626.e1.25. Lee SJ, Koh HJ. Effects of vitreomacular adhesion on anti-vascular endothelial growth factor treatment for exudative age-related macular degeneration. Ophthalmology. 2011; 118:101–110.26. Mathew R, Richardson M, Sivaprasad S. Predictive value of spectral-domain optical coherence tomography features in assessment of visual prognosis in eyes with neovascular age-related macular degeneration treated with ranibizumab. Am J Ophthalmol. 2013; 155:720–726.e1.27. Green-Simms AE, Bakri SJ. Vitreomacular traction and age-related macular degeneration. Semin Ophthalmol. 2011; 26:137–138.28. Simpson AR, Petrarca R, Jackson TL. Vitreomacular adhesion and neovascular age-related macular degeneration. Surv Ophthalmol. 2012; 57:498–509.29. Maier M, Pfrommer S, Burzer S, et al. Vitreomacular interface and posterior vitreomacular adhesion in exudative age-related macular degeneration (AMD): an OCT-based comparative study. Klin Monbl Augenheilkd. 2012; 229:1030–1035.30. Jackson TL, Nicod E, Angelis A, et al. Vitreous attachment in age-related macular degeneration, diabetic macular edema, and retinal vein occlusion: a systematic review and metaanalysis. Retina. 2013; 33:1099–1108.31. Weber-Krause B, Eckardt U. Incidence of posterior vitreous detachment in eyes with and without age-related macular degeneration: an ultrasonic study. Ophthalmologe. 1996; 93:660–665.32. Yannuzzi LA, Slakter JS, Sorenson JA, et al. Digital indocyanine green videoangiography and choroidal neovascularization. 1992. Retina. 2012; 32:Suppl 1. 191.33. Yannuzzi LA, Sorenson J, Spaide RF, Lipson B. Idiopathic polypoidal choroidal vasculopathy (IPCV). Retina. 1990; 10:1–8.34. Ross RD, Gitter KA, Cohen G, Schomaker KS. Idiopathic polypoidal choroidal vasculopathy associated with retinal arterial macroaneurysm and hypertensive retinopathy. Retina. 1996; 16:105–111.35. Stangos AN, Gandhi JS, Nair-Sahni J, et al. Polypoidal choroidal vasculopathy masquerading as neovascular age-related macular degeneration refractory to ranibizumab. Am J Ophthalmol. 2010; 150:666–673.36. Adamis AP, Shima DT. The role of vascular endothelial growth factor in ocular health and disease. Retina. 2005; 25:111–118.37. Seko Y, Seko Y, Fujikura H, et al. Induction of vascular endothelial growth factor after application of mechanical stress to retinal pigment epithelium of the rat in vitro. Invest Ophthalmol Vis Sci. 1999; 40:3287–3291.38. Meyer CH, Toth CA. Retinal pigment epithelial tear with vitreomacular attachment: a novel pathogenic feature. Graefes Arch Clin Exp Ophthalmol. 2001; 239:325–333.39. Stefansson E. Physiology of vitreous surgery. Graefes Arch Clin Exp Ophthalmol. 2009; 247:147–163.40. Reese AB, Jones IS, Cooper WC. Macular changes secondary to vitreous traction. Am J Ophthalmol. 1967; 64:Suppl. 544–549.41. Spaide RF, Armstrong D, Browne R. Continuing medical education review: choroidal neovascularization in age-related macular degeneration: what is the cause? Retina. 2003; 23:595–614.42. Rotsos T, Sagoo MS, daCruz L, et al. Intravitreal anti-VEGF treatment in eyes with combined choroidal neovascularisation and vitreomacular traction syndrome. Br J Ophthalmol. 2010; 94:1205–1210.43. Ozsutcu M, Gulkilik G, Ayintap E, et al. Intravitreal bevacizumab may increase diabetic macular edema in eyes with attached posterior vitreous. Case Rep Ophthalmol. 2013; 4:7–10.44. Manousaridis K, Talks J. Macular ischaemia: a contraindication for anti-VEGF treatment in retinal vascular disease? Br J Ophthalmol. 2012; 96:179–184.45. Mendrinos E, Mangioris G, Papadopoulou DN, et al. Long-term results of the effect of intravitreal ranibizumab on the retinal arteriolar diameter in patients with neovascular age-related macular degeneration. Acta Ophthalmol. 2013; 91:e184–e190.46. Papadopoulou DN, Mendrinos E, Mangioris G, et al. Intravitreal ranibizumab may induce retinal arteriolar vasoconstriction in patients with neovascular age-related macular degeneration. Ophthalmology. 2009; 116:1755–1761.47. Marneros AG, Fan J, Yokoyama Y, et al. Vascular endothelial growth factor expression in the retinal pigment epithelium is essential for choriocapillaris development and visual function. Am J Pathol. 2005; 167:1451–1459.48. Peters S, Heiduschka P, Julien S, et al. Ultrastructural findings in the primate eye after intravitreal injection of bevacizumab. Am J Ophthalmol. 2007; 143:995–1002.49. Shah SU, Haller JA. Vitreomacular traction in a case of exudative age-related macular degeneration resistant to anti-VEGF therapy. Acta Ophthalmol. 2012; 90:e569–e570.50. Kakinoki M, Sawada O, Sawada T, et al. Effect of vitrectomy on aqueous VEGF concentration and pharmacokinetics of bevacizumab in macaque monkeys. Invest Ophthalmol Vis Sci. 2012; 53:5877–5880.51. Christoforidis JB, Williams MM, Wang J, et al. Anatomic and pharmacokinetic properties of intravitreal bevacizumab and ranibizumab after vitrectomy and lensectomy. Retina. 2013; 33:946–952.52. Stalmans P, Benz MS, Gandorfer A, et al. Enzymatic vitreolysis with ocriplasmin for vitreomacular traction and macular holes. N Engl J Med. 2012; 367:606–615.53. Stalmans P, Duker JS, Kaiser PK, et al. Oct-based interpretation of the vitreomacular interface and indications for pharmacologic vitreolysis. Retina. 2013; 33:2003–2011.54. Anderson DH, Mullins RF, Hageman GS, Johnson LV. A role for local inflammation in the formation of drusen in the aging eye. Am J Ophthalmol. 2002; 134:411–431.55. Donoso LA, Kim D, Frost A, et al. The role of inflammation in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2006; 51:137–152.56. Zarbin MA. Current concepts in the pathogenesis of age-related macular degeneration. Arch Ophthalmol. 2004; 122:598–614.57. Ryan SJ, editor. Retina. 3rd ed. Philadelphia: Mosby;2001.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Limited Treatment Response during Follow-up after Switching to Aflibercept in Neovascular Age-related Macular Degeneration
- Course of Neovascular Age-related Macular Degeneration that Showed Limited Response to Both Ranibizumab and Aflibercept
- Efficacy of Three Aflibercept Injections for Neovascular Age-related Macular Degeneration Showing Limited Response to Ranibizumab
- Intravitreal Aflibercept for Neovascular Age-Related Macular Degeneration Resistant to Bevacizumab and Ranibizumab
- Clinical Manifestation and Result of Vitrectomy of Vitreomacular Traction Syndrome