Korean J Ophthalmol.
2015 Aug;29(4):270-279. 10.3341/kjo.2015.29.4.270.
In Vivo Effects of Preservative-free and Preserved Prostaglandin Analogs: Mouse Ocular Surface Study
- Affiliations
-
- 1Cheil Eye Research Institute, Cheil Eye Hospital, Daegu, Korea. eyepark9@naver.com
- 2Developmental Biology Laboratory, Department of Biology, College of Natural Sciences, Kyungpook National University, Daegu, Korea.
- KMID: 2363772
- DOI: http://doi.org/10.3341/kjo.2015.29.4.270
Abstract
- PURPOSE
Chronic use of topical hypotensive agents induces several side effects caused by preservatives. The purpose of this study was to evaluate the effects of prostaglandin analogs with varying concentrations of benzalkonium chloride (BAC), preservative-free (PF), and alternative preservatives on mouse corneal tissue.
METHODS
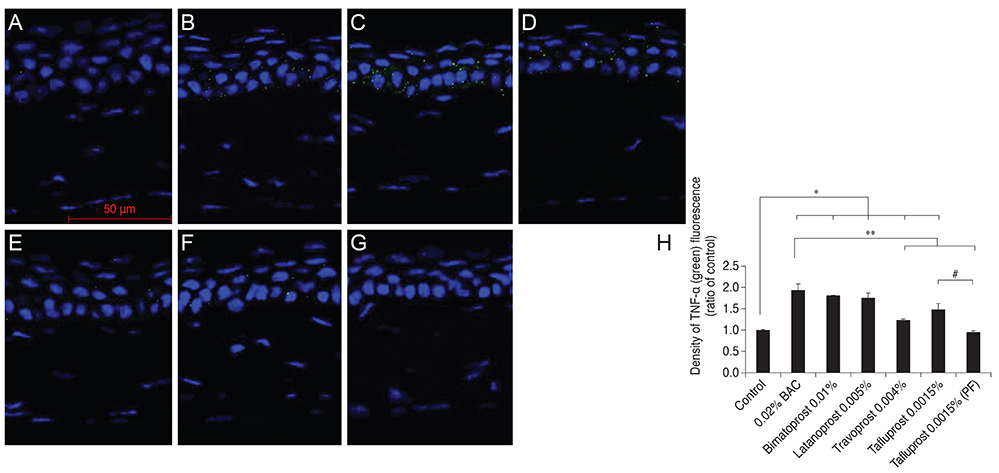
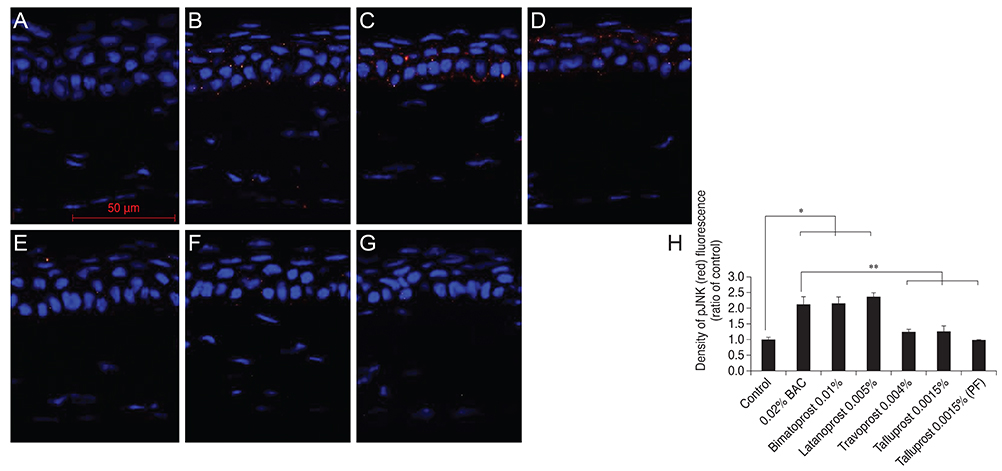
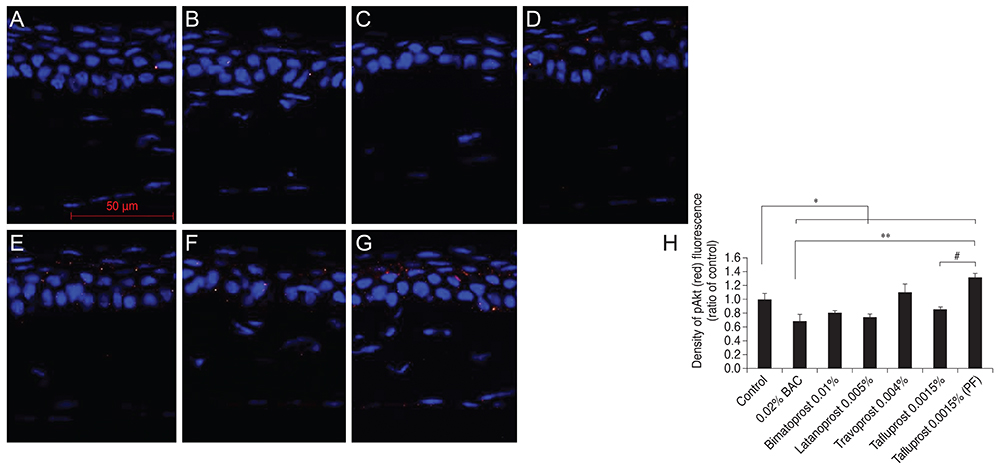
Thirty-five, 8- to 10-week-old female C57BL/6 mice (five mice for each group) were used for this study. To the control group, we applied normal saline, and to each drug-treated group we applied 0.02% BAC, bimatoprost 0.01% (with BAC 0.02%), latanoprost 0.005% (with BAC 0.02%), travoprost 0.004% (with 0.001% polyquad) or tafluprost 0.0015% with/without 0.001% BAC, once a day (9 p.m.) for 4 weeks. Corneal fluorescein staining was evaluated in all groups. After harvest, the corneal tissues were embedded in paraffin and then Hematoxylin-Eosin stain was performed for histopathological examination. Immunofluorescence staining was done against TNF-alpha, IL-6, HLA DR, pJNK, and pAkt.
RESULTS
In corneal fluorescein staining, severe punctate epithelial keratitis was seen in the groups of 0.02% BAC, 0.02% BAC containing bimatoprost 0.01% and latanoprost 0.005%. The surface desquamation, irregular surface, loss of cell borders, anisocytosis and stromal shrinkage were observed in the groups of BAC-containing eye drops. Moreover, the groups treated with BAC-containing eye drops have high inflammatory markers, significantly decreased cell viability-related signal, pAkt, and higher apoptosis-inducing signal, pJNK, than the control group. On the other hand, travoprost 0.004% and PF tafluprost 0.0015% have less cellular morphologic changes, lower inflammation, and higher cellular viability than BAC-containing formulations.
CONCLUSIONS
Corneal damage, increased inflammation and apoptosis and low cell viability were observed in BAC-containing groups. PF or alternatively preserved glaucoma medications seem to be a reasonable and viable alternative to those preserved with BAC.
MeSH Terms
-
Animals
Cell Survival
Conjunctiva/drug effects/*pathology
Disease Models, Animal
Epithelium, Corneal/drug effects/*pathology
Female
Glaucoma/*drug therapy/pathology
Mice
Mice, Inbred C57BL
Microscopy, Fluorescence
Ophthalmic Solutions
Preservatives, Pharmaceutical
Prostaglandins, Synthetic/*administration & dosage
Ophthalmic Solutions
Preservatives, Pharmaceutical
Prostaglandins, Synthetic
Figure
Reference
-
1. Goldberg I, Cunha-Vaz J, Jakobsen JE, et al. Comparison of topical travoprost eye drops given once daily and timolol 0.5% given twice daily in patients with open-angle glaucoma or ocular hypertension. J Glaucoma. 2001; 10:414–422.2. Alm A, Stjernschantz J. Effects on intraocular pressure and side effects of 0.005% latanoprost applied once daily, evening or morning: a comparison with timolol. Scandinavian Latanoprost Study Group. Ophthalmology. 1995; 102:1743–1752.3. Sherwood M, Brandt J. Bimatoprost Study Groups 1 and 2. Six-month comparison of bimatoprost once-daily and twice-daily with timolol twice-daily in patients with elevated intraocular pressure. Surv Ophthalmol. 2001; 45:Suppl 4. S361–S368.4. Broadway DC, Grierson I, O'Brien C, Hitchings RA. Adverse effects of topical antiglaucoma medication.The outcome of filtration surgery. Arch Ophthalmol. 1994; 112:1446–1454.5. Baudouin C. Allergic reaction to topical eyedrops. Curr Opin Allergy Clin Immunol. 2005; 5:459–463.6. Noecker RJ, Herrygers LA, Anwaruddin R. Corneal and conjunctival changes caused by commonly used glaucoma medications. Cornea. 2004; 23:490–496.7. Jaenen N, Baudouin C, Pouliquen P, et al. Ocular symptoms and signs with preserved and preservative-free glaucoma medications. Eur J Ophthalmol. 2007; 17:341–349.8. Brasnu E, Brignole-Baudouin F, Riancho L, et al. In vitro effects of preservative-free tafluprost and preserved latanoprost, travoprost, and bimatoprost in a conjunctival epithelial cell line. Curr Eye Res. 2008; 33:303–312.9. Baudouin C. The ocular surface in glaucoma. Cornea. 2009; 28:S14–S19.10. Baudouin C, Labbe A, Liang H, et al. Preservatives in eyedrops: the good, the bad and the ugly. Prog Retin Eye Res. 2010; 29:312–334.11. Baudouin C, Pisella PJ, Fillacier K, et al. Ocular surface inf lammatory changes induced by topical antiglaucoma drugs: human and animal studies. Ophthalmology. 1999; 106:556–563.12. Pisella PJ, Pouliquen P, Baudouin C. Prevalence of ocular symptoms and signs with preserved and preservative free glaucoma medication. Br J Ophthalmol. 2002; 86:418–423.13. Noecker R. Effects of common ophthalmic preservatives on ocular health. Adv Ther. 2001; 18:205–215.14. De Saint Jean M, Brignole F, Bringuier AF, et al. Effects of benzalkonium chloride on growth and survival of Chang conjunctival cells. Invest Ophthalmol Vis Sci. 1999; 40:619–630.15. Baudouin C, Riancho L, Warnet JM, Brignole F. In vitro studies of antiglaucomatous prostaglandin analogues: travoprost with and without benzalkonium chloride and preserved latanoprost. Invest Ophthalmol Vis Sci. 2007; 48:4123–4128.16. Guenoun JM, Baudouin C, Rat P, et al. In vitro study of inflammatory potential and toxicity profile of latanoprost, travoprost, and bimatoprost in conjunctiva-derived epithelial cells. Invest Ophthalmol Vis Sci. 2005; 46:2444–2450.17. Lee JK, Ryu YH. The effect of antiglaucoma medication on cultured human conjunctival epithelial cells. J Korean Ophthalmol Soc. 2006; 47:1811–1818.18. Liang H, Baudouin C, Pauly A, Brignole-Baudouin F. Conjunctival and corneal reactions in rabbits following short- and repeated exposure to preservative-free tafluprost, commercially available latanoprost and 0.02% benzalkonium chloride. Br J Ophthalmol. 2008; 92:1275–1282.19. Tripathi BJ, Tripathi RC, Kolli SP. Cytotoxicity of ophthalmic preservatives on human corneal epithelium. Lens Eye Toxic Res. 1992; 9:361–375.20. Asada H, Takaoka-Shichijo Y, Nakamura M, Kimura A. Optimization of benzalkonium chloride concentration in 0.0015% tafluprost ophthalmic solution from the points of ocular surface safety and preservative efficacy. Yakugaku Zasshi. 2010; 130:867–871.21. Nakagawa S, Usui T, Yokoo S, et al. Toxicity evaluation of antiglaucoma drugs using stratified human cultivated corneal epithelial sheets. Invest Ophthalmol Vis Sci. 2012; 53:5154–5160.22. Liang H, Baudouin C, Labbe A, et al. Conjunctiva-associated lymphoid tissue (CALT) reactions to antiglaucoma prostaglandins with or without BAK-preservative in rabbit acute toxicity study. PLoS One. 2012; 7:e33913.23. Liang H, Brignole-Baudouin F, Riancho L, Baudouin C. Reduced in vivo ocular surface toxicity with polyquad-preserved travoprost versus benzalkonium-preserved travoprost or latanoprost ophthalmic solutions. Ophthalmic Res. 2012; 48:89–101.24. Kim EJ, Kim YH, Kang SH, et al. In vitro effects of preservative-free and preserved prostaglandin analogs on primary cultured human conjunctival fibroblast cells. Korean J Ophthalmol. 2013; 27:446–453.25. Pauly A, Roubeix C, Liang H, et al. In vitro and in vivo comparative toxicological study of a new preservative-free latanoprost formulation. Invest Ophthalmol Vis Sci. 2012; 53:8172–8180.26. Liang H, Brignole-Baudouin F, Pauly A, et al. Polyquad-preserved travoprost/timolol, benzalkonium chloride (BAK)-preserved travoprost/timolol, and latanoprost/timolol in fixed combinations: a rabbit ocular surface study. Adv Ther. 2011; 28:311–325.27. Lu L, Wang L, Shell B. UV-induced signaling pathways associated with corneal epithelial cell apoptosis. Invest Ophthalmol Vis Sci. 2003; 44:5102–5109.28. Chen F. JNK-induced apoptosis, compensatory growth, and cancer stem cells. Cancer Res. 2012; 72:379–386.29. Niwano Y, Iwasawa A, Ayaki M. Ocular surface cytotoxicity and safety evaluation of tafluprost, a recently developed anti-glaucoma prostaglandin analog. Ophthalmol Eye Dis. 2014; 6:5–12.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Efficacy and Safety of Preservative-free Latanoprost Eyedrops Compared with Preserved Prostaglandin Analogues in Patients with Open-angle Glaucoma
- Efficacy of Preservative-free Latanoprost in Normal-tension Glaucoma with Mild to Moderate Dry Eye
- Effects of Preservative on the Meibomian Gland in Glaucoma Patients Treated with Prostaglandin Analogues
- In Vitro Effects of Preservative-free and Preserved Prostaglandin Analogs on Primary Cultured Human Conjunctival Fibroblast Cells
- Effect of the Preservative Benzalkonium Chloride in Prostaglandin Analogues on Corneal Sensitivity