Korean J Ophthalmol.
2015 Jun;29(3):160-167. 10.3341/kjo.2015.29.3.160.
The Association between Choroidal Thickness Variations and Response to Intravitreal Bevacizumab in Central Serous Chorioretinopathy
- Affiliations
-
- 1Department of Ophthalmology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. yhyoon@amc.seoul.kr
- 2Department of Ophthalmology, Gangneung Asan Hospital, University of Ulsan College of Medicine, Gangneung, Korea.
- KMID: 2363753
- DOI: http://doi.org/10.3341/kjo.2015.29.3.160
Abstract
- PURPOSE
To analyze differences in the subfoveal choroidal thickness (SFChT) between bevacizumab responders (BevRs) and nonresponders (BevNRs) in patients with idiopathic central serous chorioretinopathy (CSC).
METHODS
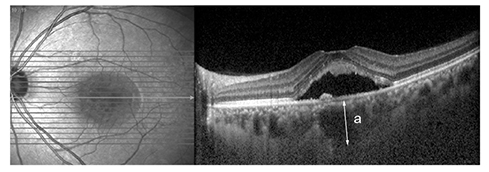
The medical records of 30 unilateral chronic CSC patients who were treated with intravitreal bevacizumab (IVB) as a first line treatment were reviewed. Patients were categorized as BevNRs when CSC did not completely resolve after a minimum of 3 IVB treatments. Enhanced depth imaging-optical coherence tomography was used and SFChT was measured before and after treatment. Choroidal hyperpermeability was also evaluated using indocyanine angiography.
RESULTS
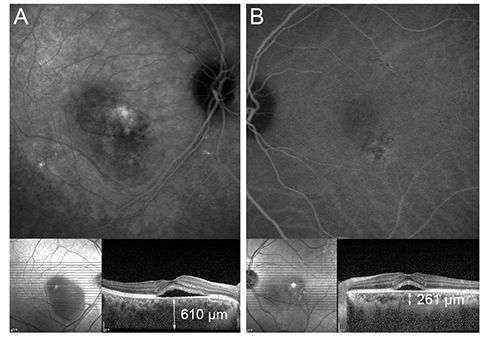
Twenty and 10 eyes were classified as BevRs or BevNRs, respectively. The mean number of IVB treatments was 2.22 +/- 0.89 in BevRs, and 4.80 +/- 1.03 in BevNRs. Compared with BevNRs, BevRs demonstrated significantly greater pretreatment SFChT (441.25 +/- 88.09 vs. 364.10 +/- 61.97 microm); SFChT reduction following IVB was significantly greater in BevRs than BevNRs. SFChT in the unaffected eyes was also greater in BevRs than BevNRs. Choroidal hyperpermeability was detected less frequently in BevNRs (hypofluorescence on late-phase, 0.0% and 33.3% in BevNRs and BevRs, respectively; p = 0.049).
CONCLUSIONS
Compared with CSC eyes that did not respond well to IVB, BevRs demonstrated significantly thicker SFChT at baseline, greater reduction in SFChT after IVB treatment, and hyperfluorescence on late-phase indocyanine green angiography. We recommend IVB injection as the first-line therapy for CSC eyes with relatively high SFChT and hyperfluorescence on late-phase indocyanine green angiography.
Keyword
MeSH Terms
-
Adult
Angiogenesis Inhibitors/administration & dosage/*therapeutic use
Bevacizumab/administration & dosage/*therapeutic use
Central Serous Chorioretinopathy/*drug therapy/pathology
Choroid/*pathology
Female
Humans
Intravitreal Injections
Male
Middle Aged
Retrospective Studies
Treatment Outcome
Angiogenesis Inhibitors
Bevacizumab
Figure
Reference
-
1. Dohrmann J, Lommatzsch A, Spital G, Pauleikhoff D. Pathogenesis of central serous chorioretinopathy: angiographic and electrophysiological studies. Ophthalmologe. 2001; 98:1069–1073.2. Piccolino FC, de la Longrais RR, Ravera G, et al. The foveal photoreceptor layer and visual acuity loss in central serous chorioretinopathy. Am J Ophthalmol. 2005; 139:87–99.3. Wong R, Chopdar A, Brown M. Five to 15 year follow-up of resolved idiopathic central serous chorioretinopathy. Eye. 2004; 18:262–268.4. Loo RH, Scott IU, Flynn HW Jr, et al. Factors associated with reduced visual acuity during long-term follow-up of patients with idiopathic central serous chorioretinopathy. Retina. 2002; 22:19–24.5. Iida T, Yannuzzi LA, Spaide RF, et al. Cystoid macular degeneration in chronic central serous chorioretinopathy. Retina. 2003; 23:1–7.6. Yannuzzi LA, Slakter JS, Gross NE, et al. Indocyanine green angiography-guided photodynamic therapy for treatment of chronic central serous chorioretinopathy: a pilot study. Retina. 2003; 23:288–298.7. Wong IY, Koizumi H, Lai WW. Enhanced depth imaging optical coherence tomography. Ophthalmic Surg Lasers Imaging. 2011; 42:S75–S84.8. Yamashita T, Yamashita T, Shirasawa M, et al. Repeatability and reproducibility of subfoveal choroidal thickness in normal eyes of Japanese using different SD-OCT devices. Invest Ophthalmol Vis Sci. 2012; 53:1102–1107.9. Branchini L, Regatieri CV, Flores-Moreno I, et al. Reproducibility of choroidal thickness measurements across three spectral domain optical coherence tomography systems. Ophthalmology. 2012; 119:119–123.10. Kuroda S, Ikuno Y, Yasuno Y, et al. Choroidal thickness in central serous chorioretinopathy. Retina. 2013; 33:302–308.11. Yang SJ, Chen CY, Chang GD, et al. Activation of Akt by advanced glycation end products (AGEs): involvement of IGF-1 receptor and caveolin-1. PLoS One. 2013; 8:e58100.12. Margolis R, Spaide RF. A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am J Ophthalmol. 2009; 147:811–815.13. Maruko I, Iida T, Sugano Y, et al. Subfoveal choroidal thickness in fellow eyes of patients with central serous chorioretinopathy. Retina. 2011; 31:1603–1608.14. Kim YT, Kang SW, Bai KH. Choroidal thickness in both eyes of patients with unilaterally active central serous chorioretinopathy. Eye (Lond). 2011; 25:1635–1640.15. Lim JW, Kim MU. The efficacy of intravitreal bevacizumab for idiopathic central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol. 2011; 249:969–974.16. Huang WC, Chen WL, Tsai YY, et al. Intravitreal bevacizumab for treatment of chronic central serous chorioretinopathy. Eye (Lond). 2009; 23:488–489.17. Schaal KB, Hoeh AE, Scheuerle A, et al. Intravitreal bevacizumab for treatment of chronic central serous chorioretinopathy. Eur J Ophthalmol. 2009; 19:613–617.18. Lim SJ, Roh MI, Kwon OW. Intravitreal bevacizumab injection for central serous chorioretinopathy. Retina. 2010; 30:100–106.19. Semeraro F, Romano MR, Danzi P, et al. Intravitreal bevacizumab versus low-f luence photodynamic therapy for treatment of chronic central serous chorioretinopathy. Jpn J Ophthalmol. 2012; 56:608–612.20. Lee YS, Ha JK, Kim YJ, et al. Comparative outcome analysis of malpositioned and properly positioned fixation groups after hamstring autograft ACL reconstruction with femoral cross-pin fixation. Knee. 2011; 18:30–33.21. Inoue R, Sawa M, Tsujikawa M, Gomi F. Association between the efficacy of photodynamic therapy and indocyanine green angiography findings for central serous chorioretinopathy. Am J Ophthalmol. 2010; 149:441–446.22. Jirarattanasopa P, Ooto S, Tsujikawa A, et al. Assessment of macular choroidal thickness by optical coherence tomography and angiographic changes in central serous chorioretinopathy. Ophthalmology. 2012; 119:1666–1678.23. Lim SH, Chang W, Sagong M. Efficacy of half-fluence photodynamic therapy depending on the degree of choroidal hyperpermeability in chronic central serous chorioretinopathy. Eye (Lond). 2013; 27:353–362.24. Cheung CS, Wong AW, Lui A, et al. Incidence of endophthalmitis and use of antibiotic prophylaxis after intravitreal injections. Ophthalmology. 2012; 119:1609–1614.25. Meyer CH, Michels S, Rodrigues EB, et al. Incidence of rhegmatogenous retinal detachments after intravitreal antivascular endothelial factor injections. Acta Ophthalmol. 2011; 89:70–75.26. Wei WB, Xu L, Jonas JB, et al. Subfoveal choroidal thickness: the Beijing Eye Study. Ophthalmology. 2013; 120:175–180.27. Usui S, Ikuno Y, Akiba M, et al. Circadian changes in subfoveal choroidal thickness and the relationship with circulatory factors in healthy subjects. Invest Ophthalmol Vis Sci. 2012; 53:2300–2307.28. Tan CS, Ouyang Y, Ruiz H, Sadda SR. Diurnal variation of choroidal thickness in normal, healthy subjects measured by spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2012; 53:261–266.29. Pryds A, Larsen M. Choroidal thickness following extrafoveal photodynamic treatment with verteporfin in patients with central serous chorioretinopathy. Acta Ophthalmol. 2012; 90:738–743.30. Barteselli G, Chhablani J, El-Emam S, et al. Choroidal volume variations with age, axial length, and sex in healthy subjects: a three-dimensional analysis. Ophthalmology. 2012; 119:2572–2578.31. Ouyang Y, Heussen FM, Mokwa N, et al. Spatial distribution of posterior pole choroidal thickness by spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2011; 52:7019–7026.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of Intravitreal Bevacizumab and Aflibercept Injections for Central Serous Chorioretinopathy
- Intravitreal Bevacizumab Injection for Choroidal Neovascularization Secondary to Laser Photocoagulation for Central Serous Chorioretinopathy
- The Short-term Effect of Intravitreal Bevacizumab for Treatment of Central Serous Chorioretinopathy
- Laser-Induced Choroidal Neovascularization in Central Serous Chorioretinopathy: 4 Cases
- Factors Influencing the Effect of the Intravitreal Bevacizumab Injection in Patients with Central Serous Chorioretinopathy