J Korean Med Sci.
2016 Apr;31(4):510-518. 10.3346/jkms.2016.31.4.510.
Pretreatment Lymphopenia, Poor Performance Status, and Early Courses of Therapy Are Risk Factors for Severe Bacterial Infection in Patients with Multiple Myeloma during Treatment with Bortezomib-based Regimens
- Affiliations
-
- 1Division of Hematology, Department of Internal Medicine, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea. hemakim@yuhs.ac
- 2Division of Infection, Department of Internal Medicine, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- 3Department of Laboratory Medicine, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2363688
- DOI: http://doi.org/10.3346/jkms.2016.31.4.510
Abstract
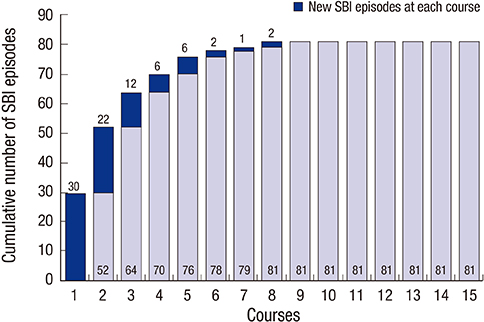
- The aim of this study was to identify the risk factors associated with severe bacterial infection (SBI) in multiple myeloma (MM) patients during treatment with bortezomib-based regimens. A total of 98 patients with MM were evaluated during 427 treatment courses. SBI occurred in 57.1% (56/98) of the patients and during 19.0% (81/427) of the treatment courses. In the multivariate analysis for the factors associated with the development of SBI in each treatment course, poor performance status (Eastern Cooperative Oncology Group ≥ 2, P < 0.001), early course of therapy (≤ 2 courses, P < 0.001), and pretreatment lymphopenia (absolute lymphocyte count < 1.0 × 10(9)/L, P = 0.043) were confirmed as independent risk factors. The probability of developing SBI were 5.1%, 14.9%, 23.9% and 59.5% in courses with 0, 1, 2, and 3 risk factors, respectively (P < 0.001). In conclusion, we identified three pretreatment risk factors associated with SBI in each course of bortezomib treatment. Therefore, MM patients with these risk factors should be more closely monitored for the development of SBI during bortezomib-based treatment.
Keyword
MeSH Terms
-
Adult
Aged
Aged, 80 and over
Antineoplastic Combined Chemotherapy Protocols/therapeutic use
Bacterial Infections/*complications/microbiology
Bortezomib/*administration & dosage
Female
Gram-Negative Bacteria/isolation & purification
Gram-Positive Bacteria/isolation & purification
Humans
Lymphocyte Count
Lymphopenia/*therapy
Male
Middle Aged
Multiple Myeloma/complications/*drug therapy/mortality
Multivariate Analysis
Proportional Hazards Models
Retrospective Studies
Risk Factors
Stem Cell Transplantation
Survival Rate
Transplantation, Homologous
Bortezomib
Figure
Reference
-
1. Augustson BM, Begum G, Dunn JA, Barth NJ, Davies F, Morgan G, Behrens J, Smith A, Child JA, Drayson MT. Early mortality after diagnosis of multiple myeloma: analysis of patients entered onto the United Kingdom Medical Research Council trials between 1980 and 2002--Medical Research Council Adult Leukaemia Working Party. J Clin Oncol. 2005; 23:9219–9226.2. Blimark C, Holmberg E, Mellqvist UH, Landgren O, Björkholm M, Hultcrantz M, Kjellander C, Turesson I, Kristinsson SY. Multiple myeloma and infections: a population-based study on 9253 multiple myeloma patients. Haematologica. 2015; 100:107–113.3. Kuroki Y, Tsuchida K, Go I, Aoyama M, Naganuma T, Takemoto Y, Nakatani T. A study of innate immunity in patients with end-stage renal disease: special reference to toll-like receptor-2 and -4 expression in peripheral blood monocytes of hemodialysis patients. Int J Mol Med. 2007; 19:783–790.4. Bodey GP, Buckley M, Sathe YS, Freireich EJ. Quantitative relationships between circulating leukocytes and infection in patients with acute leukemia. Ann Intern Med. 1966; 64:328–340.5. Bow EJ. Infection in neutropenic patients with cancer. Crit Care Clin. 2013; 29:411–441.6. Stuck AE, Minder CE, Frey FJ. Risk of infectious complications in patients taking glucocorticosteroids. Rev Infect Dis. 1989; 11:954–963.7. Teh BW, Harrison SJ, Pellegrini M, Thursky KA, Worth LJ, Slavin MA. Changing treatment paradigms for patients with plasma cell myeloma: impact upon immune determinants of infection. Blood Rev. 2014; 28:75–86.8. Cerundolo V, Benham A, Braud V, Mukherjee S, Gould K, Macino B, Neefjes J, Townsend A. The proteasome-specific inhibitor lactacystin blocks presentation of cytotoxic T lymphocyte epitopes in human and murine cells. Eur J Immunol. 1997; 27:336–341.9. Berges C, Haberstock H, Fuchs D, Miltz M, Sadeghi M, Opelz G, Daniel V, Naujokat C. Proteasome inhibition suppresses essential immune functions of human CD4+ T cells. Immunology. 2008; 124:234–246.10. Basler M, Lauer C, Beck U, Groettrup M. The proteasome inhibitor bortezomib enhances the susceptibility to viral infection. J Immunol. 2009; 183:6145–6150.11. San Miguel JF, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M, Spicka I, Petrucci MT, Palumbo A, Samoilova OS, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008; 359:906–917.12. Chanan-Khan A, Sonneveld P, Schuster MW, Stadtmauer EA, Facon T, Harousseau JL, Ben-Yehuda D, Lonial S, Goldschmidt H, Reece D, et al. Analysis of herpes zoster events among bortezomib-treated patients in the phase III APEX study. J Clin Oncol. 2008; 26:4784–4790.13. Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, Facon T, Harousseau JL, Ben-Yehuda D, Lonial S, Goldschmidt H, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005; 352:2487–2498.14. Facon T, Mary JY, Hulin C, Benboubker L, Attal M, Pegourie B, Renaud M, Harousseau JL, Guillerm G, Chaleteix C, et al. Melphalan and prednisone plus thalidomide versus melphalan and prednisone alone or reduced-intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99-06): a randomised trial. Lancet. 2007; 370:1209–1218.15. Facon T, Mary JY, Pégourie B, Attal M, Renaud M, Sadoun A, Voillat L, Dorvaux V, Hulin C, Lepeu G, et al. Dexamethasone-based regimens versus melphalan-prednisone for elderly multiple myeloma patients ineligible for high-dose therapy. Blood. 2006; 107:1292–1298.16. Cesana C, Nosari AM, Klersy C, Miqueleiz S, Rossi V, Ferrando P, Valentini M, Barbarano L, Morra E. Risk factors for the development of bacterial infections in multiple myeloma treated with two different vincristine-adriamycin-dexamethasone schedules. Haematologica. 2003; 88:1022–1028.17. Segeren CM, Sonneveld P, van der Holt B, Baars JW, Biesma DH, Cornellissen JJ, Croockewit AJ, Dekker AW, Fibbe WE, Löwenberg B, et al. Vincristine, doxorubicin and dexamethasone (VAD) administered as rapid intravenous infusion for first-line treatment in untreated multiple myeloma. Br J Haematol. 1999; 105:127–130.18. Corso A, Barbarano L, Zappasodi P, Cairoli R, Alessandrino EP, Mangiacavalli S, Ferrari D, Fava S, Fiumanò M, Frigerio G, et al. The VAD-DCEP sequence is an effective pre-transplant therapy in untreated multiple myeloma. Haematologica. 2004; 89:1124–1127.19. Durie BG, Harousseau JL, Miguel JS, Bladé J, Barlogie B, Anderson K, Gertz M, Dimopoulos M, Westin J, Sonneveld P, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006; 20:1467–1473.20. Pruzanski W, Gidon MS, Roy A. Suppression of polyclonal immunoglobulins in multiple myeloma: relationship to the staging and other manifestations at diagnosis. Clin Immunol Immunopathol. 1980; 17:280–286.21. Kastritis E, Zagouri F, Symeonidis A, Roussou M, Sioni A, Pouli A, Delimpasi S, Katodritou E, Michalis E, Michael M, et al. Preserved levels of uninvolved immunoglobulins are independently associated with favorable outcome in patients with symptomatic multiple myeloma. Leukemia. 2014; 28:2075–2079.22. Perri RT, Hebbel RP, Oken MM. Influence of treatment and response status on infection risk in multiple myeloma. Am J Med. 1981; 71:935–940.23. Mainwaring CJ, Williams MA, Singer CR, Lush RJ, Smith JG, Haynes CL, Kelsey SM. Monocyte dysfunction in patients with multiple myeloma and lymphoplasmacytic disorders is related to serum paraprotein levels. Br J Haematol. 1999; 105:948–954.24. Takamatsu H, Honda S, Miyamoto T, Yokoyama K, Hagiwara S, Ito T, Tomita N, Iida S, Iwasaki T, Sakamaki H, et al. Changing trends in prognostic factors for patients with multiple myeloma after autologous stem cell transplantation during the immunomodulator drug/proteasome inhibitor era. Cancer Sci. 2015; 106:179–185.25. Yoshikawa TT. Epidemiology and unique aspects of aging and infectious diseases. Clin Infect Dis. 2000; 30:931–933.26. Savage DG, Lindenbaum J, Garrett TJ. Biphasic pattern of bacterial infection in multiple myeloma. Ann Intern Med. 1982; 96:47–50.27. Howard A, O’Donoghue M, Feeney A, Sleator RD. Acinetobacter baumannii: an emerging opportunistic pathogen. Virulence. 2012; 3:243–250.28. Yi YS, Chung JS, Song MK, Shin HJ, Seol YM, Choi YJ, Cho GJ, Lee GW, Moon JH, Hwang IH, et al. The risk factors for herpes zoster in bortezomib treatment in patients with multiple myeloma. Korean J Hematol. 2010; 45:188–192.29. de Jager CP, van Wijk PT, Mathoera RB, de Jongh-Leuvenink J, van der Poll T, Wever PC. Lymphocytopenia and neutrophil-lymphocyte count ratio predict bacteremia better than conventional infection markers in an emergency care unit. Crit Care. 2010; 14:R192.30. Jung SH, Bae SY, Ahn JS, Kang SJ, Yang DH, Kim YK, Kim HJ, Lee JJ. Lymphocytopenia is associated with an increased risk of severe infections in patients with multiple myeloma treated with bortezomib-based regimens. Int J Hematol. 2013; 97:382–387.31. Klein NC, Go CH, Cunha BA. Infections associated with steroid use. Infect Dis Clin North Am. 2001; 15:423–432.32. Rajkumar SV, Jacobus S, Callander NS, Fonseca R, Vesole DH, Williams ME, Abonour R, Siegel DS, Katz M, Greipp PR; Eastern Cooperative Oncology Group. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: an open-label randomised controlled trial. Lancet Oncol. 2010; 11:29–37.33. Vesole DH, Oken MM, Heckler C, Greipp PR, Katz MS, Jacobus S, Morrow GR; University of Rochester Cancer Center and the Eastern Cooperative Oncology Group. Oral antibiotic prophylaxis of early infection in multiple myeloma: a URCC/ECOG randomized phase III study. Leukemia. 2012; 26:2517–2520.34. National Comprehensive Cancer Network (US). NCCN clinical practice guidelines in oncology: prevention and treatment of cancer-related infections, version 2 [Internet]. Fort Washington, PA: National Comprehensive Cancer Network;2015. accessed on 10 August 2015. Available at http://www.nccn.org/professionals/physician_gls/pdf/infections.pdf.35. Wittekamp BH, Bonten MJ. Antibiotic prophylaxis in the era of multidrug-resistant bacteria. Expert Opin Investig Drugs. 2012; 21:767–772.36. Teh BW, Teng JC, Urbancic K, Grigg A, Harrison SJ, Worth LJ, Slavin MA, Thursky KA. Invasive fungal infections in patients with multiple myeloma: a multi-center study in the era of novel myeloma therapies. Haematologica. 2015; 100:e28–31.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Acute Pancreatitis Caused by Bortezomib in a Patient with Multiple Myeloma
- Reversible Heart Failure after Bortezomib Treatment in a Patient with Multiple Myeloma
- Bortezomib Induced Tumor Lysis Syndrome in Multiple Myeloma
- Poor prognostic significance of Mycobacterium tuberculosis infection during bortezomib-containing chemotherapy in patients with multiple myeloma
- A Case of Cutaneous Plasmacytoma Treated with Bortezomib and Radiotherapy