J Korean Med Sci.
2016 Mar;31(3):360-370. 10.3346/jkms.2016.31.3.360.
Dual-Blocking of PI3K and mTOR Improves Chemotherapeutic Effects on SW620 Human Colorectal Cancer Stem Cells by Inducing Differentiation
- Affiliations
-
- 1Department of Biotechnology, College of Life Sciences and Biotechnology, Korea University, Seoul, Korea. cwkim@korea.ac.kr
- 2Ministry of Food and Drug Safety, Cheongju, Korea.
- KMID: 2363495
- DOI: http://doi.org/10.3346/jkms.2016.31.3.360
Abstract
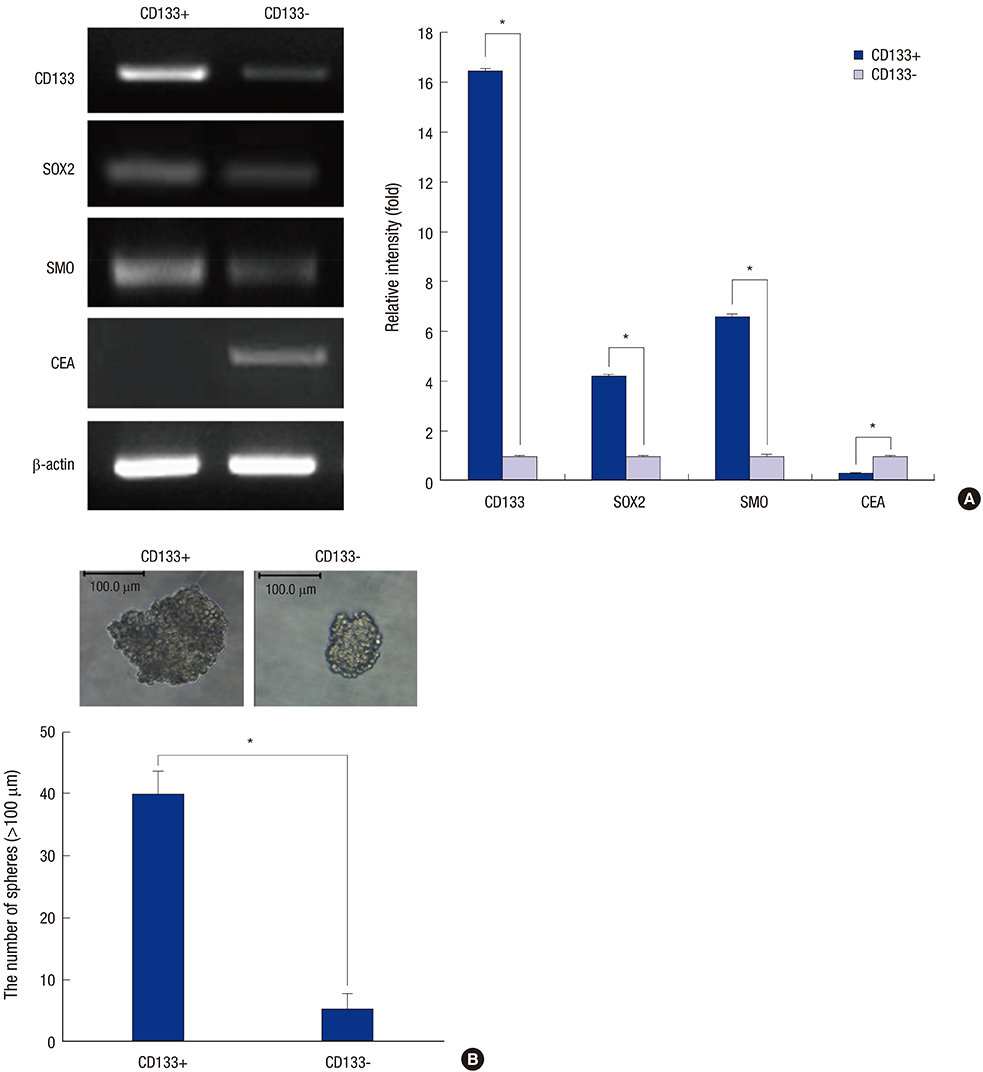
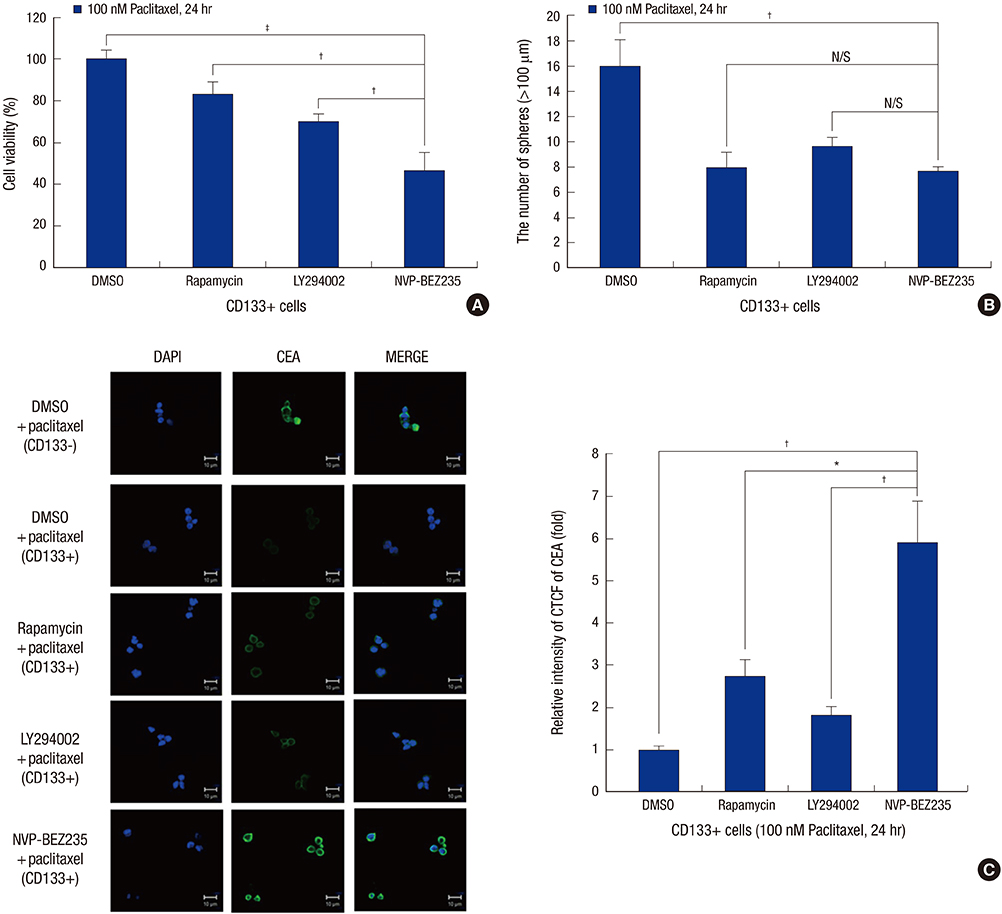
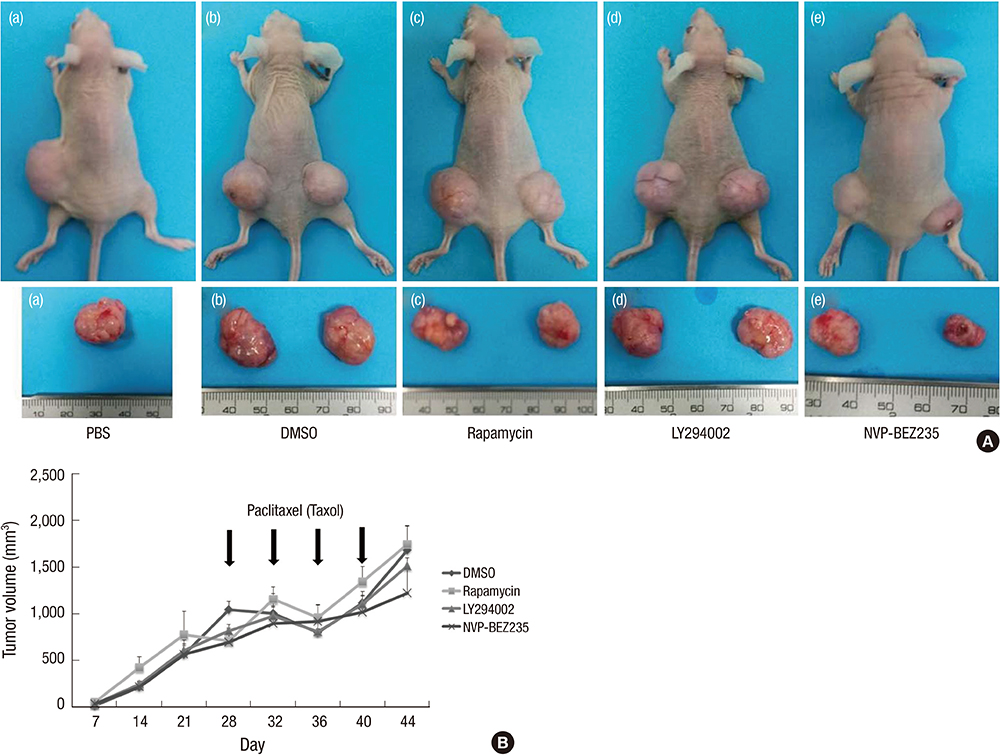
- Cancer stem cells (CSCs) have tumor initiation, self-renewal, metastasis and chemo-resistance properties in various tumors including colorectal cancer. Targeting of CSCs may be essential to prevent relapse of tumors after chemotherapy. Phosphatidylinositol-3-kinase (PI3K) and mammalian target of rapamycin (mTOR) signals are central regulators of cell growth, proliferation, differentiation, and apoptosis. These pathways are related to colorectal tumorigenesis. This study focused on PI3K and mTOR pathways by inhibition which initiate differentiation of SW620 derived CSCs and investigated its effect on tumor progression. By using rapamycin, LY294002, and NVP-BEZ235, respectively, PI3K and mTOR signals were blocked independently or dually in colorectal CSCs. Colorectal CSCs gained their differentiation property and lost their stemness properties most significantly in dual-blocked CSCs. After treated with anti-cancer drug (paclitaxel) on the differentiated CSCs cell viability, self-renewal ability and differentiation status were analyzed. As a result dual-blocking group has most enhanced sensitivity for anti-cancer drug. Xenograft tumorigenesis assay by using immunodeficiency mice also shows that dual-inhibited group more effectively increased drug sensitivity and suppressed tumor growth compared to single-inhibited groups. Therefore it could have potent anti-cancer effects that dual-blocking of PI3K and mTOR induces differentiation and improves chemotherapeutic effects on SW620 human colorectal CSCs.
Keyword
MeSH Terms
-
AC133 Antigen/genetics/metabolism
Animals
Antineoplastic Agents/pharmacology/therapeutic use
Cell Differentiation/*drug effects
Cell Line, Tumor
Cell Survival/drug effects
Chromones/pharmacology/therapeutic use
Colorectal Neoplasms/drug therapy/metabolism/pathology
Humans
Imidazoles/pharmacology/therapeutic use
Male
Mice
Mice, Inbred BALB C
Mice, Nude
Morpholines/pharmacology/therapeutic use
Neoplastic Stem Cells/cytology/drug effects/metabolism
Paclitaxel/pharmacology/therapeutic use
Phosphatidylinositol 3-Kinases/*antagonists & inhibitors/metabolism
Quinolines/pharmacology/therapeutic use
SOXB1 Transcription Factors/genetics/metabolism
Signal Transduction/*drug effects
Sirolimus/pharmacology/therapeutic use
TOR Serine-Threonine Kinases/*antagonists & inhibitors/metabolism
Xenograft Model Antitumor Assays
AC133 Antigen
Antineoplastic Agents
Chromones
Imidazoles
Morpholines
Paclitaxel
Phosphatidylinositol 3-Kinases
Quinolines
SOXB1 Transcription Factors
Sirolimus
TOR Serine-Threonine Kinases
Figure
Reference
-
1. Lee EK, Han GY, Park HW, Song YJ, Kim CW. Transgelin promotes migration and invasion of cancer stem cells. J Proteome Res. 2010; 9:5108–5117.2. Jordan CT, Guzman ML, Noble M. Cancer stem cells. N Engl J Med. 2006; 355:1253–1261.3. Lobo NA, Shimono Y, Qian D, Clarke MF. The biology of cancer stem cells. Annu Rev Cell Dev Biol. 2007; 23:675–699.4. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012; 62:10–29.5. Botchkina G. Colon cancer stem cells--from basic to clinical application. Cancer Lett. 2013; 338:127–140.6. O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007; 445:106–110.7. Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007; 445:111–115.8. Alison MR, Lin WR, Lim SM, Nicholson LJ. Cancer stem cells: in the line of fire. Cancer Treat Rev. 2012; 38:589–598.9. Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002; 2:489–501.10. Osaki M, Oshimura M, Ito H. PI3K-Akt pathway: its functions and alterations in human cancer. Apoptosis. 2004; 9:667–676.11. Katso R, Okkenhaug K, Ahmadi K, White S, Timms J, Waterfield MD. Cellular function of phosphoinositide 3-kinases: implications for development, homeostasis, and cancer. Annu Rev Cell Dev Biol. 2001; 17:615–675.12. Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov. 2009; 8:627–644.13. Pandurangan AK. Potential targets for prevention of colorectal cancer: a focus on PI3K/Akt/mTOR and Wnt pathways. Asian Pac J Cancer Prev. 2013; 14:2201–2205.14. Slattery ML, Herrick JS, Lundgreen A, Fitzpatrick FA, Curtin K, Wolff RK. Genetic variation in a metabolic signaling pathway and colon and rectal cancer risk: mTOR, PTEN, STK11, RPKAA1, PRKAG2, TSC1, TSC2, PI3K and Akt1. Carcinogenesis. 2010; 31:1604–1611.15. Choi KS, Shin JS, Lee JJ, Kim YS, Kim SB, Kim CW. In vitro trans-differentiation of rat mesenchymal cells into insulin-producing cells by rat pancreatic extract. Biochem Biophys Res Commun. 2005; 330:1299–1305.16. Dontu G, Al-Hajj M, Abdallah WM, Clarke MF, Wicha MS. Stem cells in normal breast development and breast cancer. Cell Prolif. 2003; 36:Suppl 1. 59–72.17. Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007; 449:1003–1007.18. Kim DD, Eng C. The promise of mTOR inhibitors in the treatment of colorectal cancer. Expert Opin Investig Drugs. 2012; 21:1775–1788.19. Takeuchi H, Kondo Y, Fujiwara K, Kanzawa T, Aoki H, Mills GB, Kondo S. Synergistic augmentation of rapamycin-induced autophagy in malignant glioma cells by phosphatidylinositol 3-kinase/protein kinase B inhibitors. Cancer Res. 2005; 65:3336–3346.20. Fan QW, Knight ZA, Goldenberg DD, Yu W, Mostov KE, Stokoe D, Shokat KM, Weiss WA. A dual PI3 kinase/mTOR inhibitor reveals emergent efficacy in glioma. Cancer Cell. 2006; 9:341–349.21. Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer. 2009; 9:550–562.22. Palozza P, Torelli C, Boninsegna A, Simone R, Catalano A, Mele MC, Picci N. Growth-inhibitory effects of the astaxanthin-rich alga Haematococcus pluvialis in human colon cancer cells. Cancer Lett. 2009; 283:108–117.23. Yatscoff RW, LeGatt DF, Kneteman NM. Therapeutic monitoring of rapamycin: a new immunosuppressive drug. Ther Drug Monit. 1993; 15:478–482.24. Sabatini DM. mTOR and cancer: insights into a complex relationship. Nat Rev Cancer. 2006; 6:729–734.25. Faivre S, Kroemer G, Raymond E. Current development of mTOR inhibitors as anticancer agents. Nat Rev Drug Discov. 2006; 5:671–688.26. Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007; 12:9–22.27. Hay N. The Akt-mTOR tango and its relevance to cancer. Cancer Cell. 2005; 8:179–183.28. Koehl GE, Spitzner M, Ousingsawat J, Schreiber R, Geissler EK, Kunzelmann K. Rapamycin inhibits oncogenic intestinal ion channels and neoplasia in APC(Min/+) mice. Oncogene. 2010; 29:1553–1560.29. Maira SM, Stauffer F, Brueggen J, Furet P, Schnell C, Fritsch C, Brachmann S, Chène P, De Pover A, Schoemaker K, et al. Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol Cancer Ther. 2008; 7:1851–1863.30. Serra V, Markman B, Scaltriti M, Eichhorn PJ, Valero V, Guzman M, Botero ML, Llonch E, Atzori F, Di Cosimo S, et al. NVP-BEZ235, a dual PI3K/mTOR inhibitor, prevents PI3K signaling and inhibits the growth of cancer cells with activating PI3K mutations. Cancer Res. 2008; 68:8022–8030.31. Fang DD, Zhang CC, Gu Y, Jani JP, Cao J, Tsaparikos K, Yuan J, Thiel M, Jackson-Fisher A, Zong Q, et al. Antitumor efficacy of the dual PI3K/mTOR inhibitor PF-04691502 in a human xenograft tumor model derived from colorectal cancer stem cells Harboring a Mutation. PLoS One. 2013; 8:e67258.32. Lin SJ, Leng ZG, Guo YH, Cai L, Cai Y, Li N, Shang HB, Le WD, Zhao WG, Wu ZB. Suppression of mTOR pathway and induction of autophagy-dependent cell death by cabergoline. Oncotarget. 2015; 6:39329–39341.33. Petrelli A, Carollo R, Cargnelutti M, Iovino F, Callari M, Cimino D, Todaro M, Mangiapane LR, Giammona A, Cordova A, et al. By promoting cell differentiation, miR-100 sensitizes basal-like breast cancer stem cells to hormonal therapy. Oncotarget. 2015; 6:2315–2330.34. Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005; 5:275–284.35. Rich JN. Cancer stem cells in radiation resistance. Cancer Res. 2007; 67:8980–8984.36. Tang C, Ang BT, Pervaiz S. Cancer stem cell: target for anti-cancer therapy. FASEB J. 2007; 21:3777–3785.37. Dubrovska A, Elliott J, Salamone RJ, Kim S, Aimone LJ, Walker JR, Watson J, Sauveur-Michel M, Garcia-Echeverria C, Cho CY, et al. Combination therapy targeting both tumor-initiating and differentiated cell populations in prostate carcinoma. Clin Cancer Res. 2010; 16:5692–5702.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Myricetin Inhibits Angiogenesis by Inducing Apoptosis and Suppressing PI3K/Akt/mTOR Signaling in Endothelial Cells
- Dioscin Decreases Breast Cancer Stem-like Cell Proliferation via Cell Cycle Arrest by Modulating p38 Mitogen-activated Protein Kinase and AKT/mTOR Signaling Pathways
- Application of Bone Marrow Mesenchymal Stem Cells Effectively Eliminates Endotoxemia to Protect Rat from Acute Liver Failure Induced by Thioacetamide
- Roles of mTOR Signaling in Brain Development
- Induction of Hepatocellular Carcinoma Cell Cycle Arrest and Apoptosis by Dendropanax morbifera Leveille Leaf Extract via the PI3K/AKT/mTOR Pathway