J Vet Sci.
2016 Mar;17(1):115-117. 10.4142/jvs.2016.17.1.115.
Viscerotropic velogenic Newcastle disease virus replication in feathers of infected chickens
- Affiliations
-
- 1Avian Disease Laboratory, College of Veterinary Medicine, Konkuk University, Seoul 05029, Korea. songcs@konkuk.ac.kr
- 2Laboratory of Veterinary Anatomy, College of Veterinary Medicine, Konkuk University, Seoul 05029, Korea.
- 3Animal and Plant Quarantine Agency, Ministry of Agriculture, Food and Rural Affairs, Anyang 14089, Korea.
- KMID: 2363343
- DOI: http://doi.org/10.4142/jvs.2016.17.1.115
Abstract
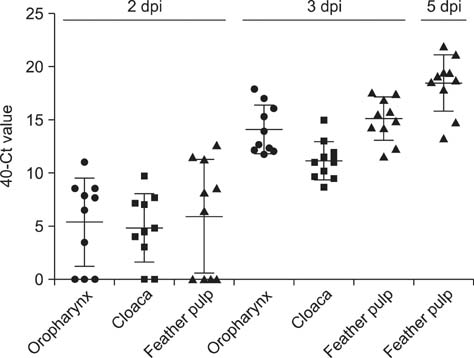
- Newcastle disease viruses (NDVs) cause systemic diseases in chickens with high mortality. However, little is known about persistence of NDVs in contaminated tissues from infected birds. In this study, we examined viral replication in the feather pulp of chickens inoculated with viscerotropic velogenic NDV (vvNDV) genotype VII. Reverse transcription real-time PCR and immunohistochemistry were used to investigate viral persistence in the samples. vvNDV was detected in the oropharynx and cloaca and viral antigens were detected in the feathers, suggesting that feathers act as sources of viral transmission.
Keyword
MeSH Terms
Figure
Reference
-
1. Leslie J. Newcastle disease: outbreak losses and control policy costs. Vet Rec. 2000; 146:603–606.
Article2. Mayo MA. Virus taxonomy - Houston 2002. Arch Virol. 2002; 147:1071–1076.3. Saif YM, Fadly AM, Glisson JR, McDougald LR, Nolan LK, Swayne DE. Diseases of poultry. 12th ed. Ames: Blackwell Publishing Professional;2008. p. 75–100.4. Wise MG, Suarez DL, Seal BS, Pedersen JC, Senne DA, King DJ, Kapczynski DR, Spackman E. Development of a real-time reverse-transcription PCR for detection of Newcastle disease virus RNA in clinical samples. J Clin Microbiol. 2004; 42:329–338.
Article5. Yamamoto Y, Nakamura K, Okamatsu M, Miyazaki A, Yamada M, Mase M. Detecting avian influenza virus (H5N1) in domestic duck feathers. Emerg Infect Dis. 2008; 14:1671–1672.
Article6. Yamamoto Y, Nakamura K, Okamatsu M, Yamada M, Mase M. Avian influenza virus (H5N1) replication in feathers of domestic waterfowl. Emerg Infect Dis. 2008; 14:149–151.
Article7. Yamamoto Y, Nakamura K, Yamada M, Ito T. Zoonotic risk for influenza A (H5N1) infection in wild swan feathers. J Vet Med Sci. 2009; 71:1549–1551.
Article8. Yamamoto Y, Nakamura K, Yamada M, Mase M. Persistence of avian influenza virus (H5N1) in feathers detached from bodies of infected domestic ducks. Appl Environ Microbiol. 2010; 76:5496–5499.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Red blood cell elution time of strains of Newcastle disease virus
- Characterization and comparison of the pathogenicity of viscerotropic velogenic Newcastle disease virus isolates in Korea
- Characterisation of genotype VII Newcastle disease virus (NDV) isolated from NDV vaccinated chickens, and the efficacy of LaSota and recombinant genotype VII vaccines against challenge with velogenic NDV
- Protection of chickens from Newcastle disease with a recombinant baculovirus subunit vaccine expressing the fusion and hemagglutininneuraminidase proteins
- Immunosuppressive effect of Cryptosporidium baileyi infection on vaccination against Newcastle disease in chicks