J Vet Sci.
2016 Mar;17(1):27-34. 10.4142/jvs.2016.17.1.27.
Assessment of the safety and efficacy of low pathogenic avian influenza (H9N2) virus in inactivated oil emulsion vaccine in laying hens
- Affiliations
-
- 1Environmental Health Research Division, National Institute of Environmental Research, Incheon 22689, Korea.
- 2Avian Diseases Laboratory, College of Veterinary Medicine, Kangwon National University, Chuncheon 24341, Korea.
- 3Avian Diseases Laboratory, College of Veterinary Medicine, Chungbuk National University, Cheongju 28665, Korea.
- 4Ha Veterinary Clinics, Yeongju 36082, Korea. ha9975@hanmail.net
- KMID: 2363332
- DOI: http://doi.org/10.4142/jvs.2016.17.1.27
Abstract
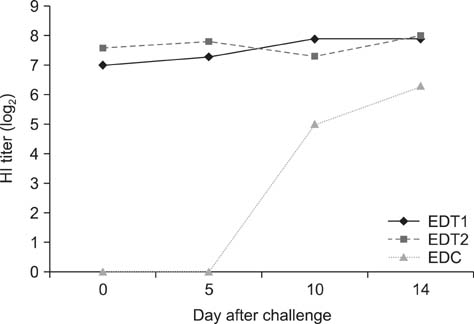
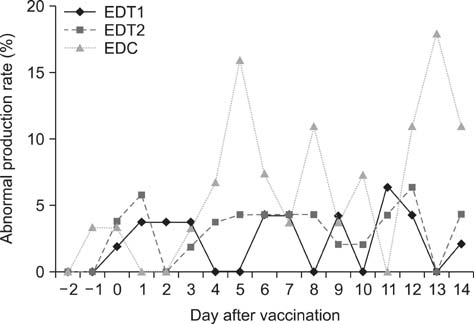
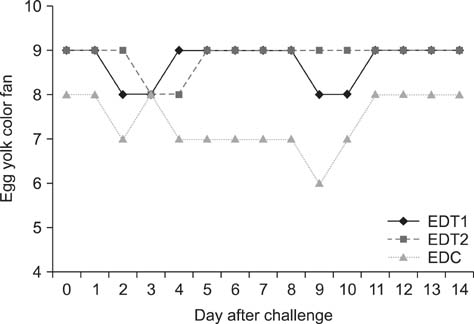
- In Korea, several outbreaks of low pathogenic AI (H9N2) viral infections leading to decreased egg production and increased mortality have been reported on commercial farms since 1996, resulting in severe economic losses. To control the H9N2 LPAI endemic, the Korea Veterinary Authority has permitted the use of the inactivated H9N2 LPAI vaccine since 2007. In this study, we developed a killed vaccine using a low pathogenic H9N2 AI virus (A/chicken/Korea/ADL0401) and conducted safety and efficacy tests in commercial layer farms while focusing on analysis of factors that cause losses to farms, including egg production rate, egg abnormality, and feed efficiency. The egg production rate of the control group declined dramatically 5 days after the challenge. There were no changes in feed consumption of all three groups before the challenge, but rates of the control declined afterward. Clinical signs in the vaccinated groups were similar, and a slight decline in feed consumption was observed after challenge; however, this returned to normal more rapidly than the control group and commercial layers. Overall, the results of this study indicate that the safety and efficacy of the vaccine are adequate to provide protection against the AI field infection (H9N2) epidemic in Korea.
Keyword
MeSH Terms
Figure
Reference
-
1. Allan WH, Lancaster JE, Tóth B. Newcastle Disease Vaccines: Their Production and Use. Rome: Food and Agriculture Organization of the United Nations;1978. p. 54–94.2. Banks J, Speidel EC, Harris PA, Alexander DJ. Phylogenetic analysis of influenza A viruses of H9 haemagglutinin subtype. Avian Pathol. 2000; 29:353–359.
Article3. Beard CW, Schnitzlein WM, Tripathy DN. Protection of chickens against highly pathogenic avian influenza virus (H5N2) by recombinant fowlpox viruses. Avian Dis. 1991; 35:356–359.
Article4. Breytenbach JH. Vaccination and biosecurity is the key. Poult World. 2005; 159:33.5. Capua I, Mutinelli F, Marangon S, Alexander DJ. H7N1 avian influenza in Italy (1999 to 2000) in intensively reared chickens and turkeys. Avian Pathol. 2000; 29:537–543.
Article6. Chen H, Subbarao K, Swayne D, Chen Q, Lu X, Katz J, Cox N, Matsuoka Y. Generation and evaluation of a high-growth reassortant H9N2 influenza A virus as a pandemic vaccine candidate. Vaccine. 2003; 21:1974–1979.
Article7. Choi JG, Lee YJ, Kim YJ, Lee EK, Jeong OM, Sung HW, Kim JH, Kwon JH. An inactivated vaccine to control the current H9N2 low pathogenic avian influenza in Korea. J Vet Sci. 2008; 9:67–74.
Article8. Crinion RAP. Egg quality and production following infectious bronchitis virus exposure at one day old. Poult Sci. 1972; 51:582–585.
Article9. Crinion RAP, Ball RA, Hofstad MS. Abnormalities in laying chickens following exposure to infectious bronchitis virus at one day old. Avian Dis. 1971; 15:42–48.
Article10. De BK, Shaw MW, Rota PA, Harmon MW, Esposito JJ, Rott R, Cox NJ, Kendal AP. Protection against virulent H5 avian influenza virus infection in chickens by an inactivated vaccine produced with recombinant vaccinia virus. Vaccine. 1988; 6:257–261.
Article11. Di Trani L, Cordioli P, Falcone E, Lombardi G, Moreno A, Sala G, Tollis M. Standardization of an inactivated H17N1 avian influenza vaccine and efficacy against A/Chicken/Italy/13474/99 high-pathogenicity virus infection. Avian Dis. 2003; 47:Suppl 3. 1042–1046.
Article12. Hitchner SB. Virus propagation in embryonating eggs. In : Hitchner SB, Domermuth CH, Purchase HG, Williams JE, editors. Isolation and Identification of Avian Pathogens. 2nd ed. College Station: American Association of Avian Pathologists;1980. p. 120–121.13. Karunakaran D, Newman JA, Halvorson DA, Abraham A. Evaluation of inactivated influenza vaccines in market turkeys. Avian Dis. 1987; 31:498–503.
Article14. Lee CH, Byun SH, Lee YJ, Mo IP. Genetic evolution of the H9N2 avian influenza virus in Korean poultry farms. Virus Genes. 2012; 45:38–47.
Article15. Lee CW, Song CS, Lee YJ, Mo IP, Garcia M, Suarez DL, Kim SJ. Sequence analysis of the hemagglutinin gene of H9N2 Korean avian influenza viruses and assessment of the pathogenic potential of isolate MS96. Avian Dis. 2000; 44:527–535.
Article16. Lee DH, Song CS. H9N2 avian influenza virus in Korea: evolution and vaccination. Clin Exp Vaccine Res. 2013; 2:26–33.
Article17. Lee HJ, Kwon JS, Lee DH, Lee YN, Youn HN, Lee YJ, Kim MC, Jeong OM, Kang HM, Kwon JH, Lee JB, Park SY, Choi IS, Song CS. Continuing evolution and interspecies transmission of influenza viruses in live bird markets in Korea. Avian Dis. 2010; 54:Suppl 1. 738–748.
Article18. Lu H, Castro AE, Pennick K, Liu J, Yang Q, Dunn P, Weinstock D, Henzler D. Survival of avian influenza virus H7N2 in SPF chickens and their environments. Avian Dis. 2003; 47:Suppl 3. 1015–1021.
Article19. Lu X, Edwards LE, Desheva JA, Nguyen DC, Rekstin A, Stephenson I, Szretter K, Cox NJ, Rudenko LG, Klimov A, Katz JM. Cross-protective immunity in mice induced by live-attenuated or inactivated vaccines against highly pathogenic influenza A (H5N1) viruses. Vaccine. 2006; 24:6588–6593.
Article20. Marangon S, Bortolotti L, Capua I, Bettio M, Dalla Pozza M. Low-pathogenicity avian influenza (LPAI) in Italy (2000-01): epidemiology and control. Avian Dis. 2003; 47:Suppl 3. 1006–1009.
Article21. Mo IP, Song CS, Kim KS, Rhee JC. An occurrence of non-highly pathogenic avian influenza in Korea. In : Proceedings of the Forth International Symposium on Avian Influenza; 29-31 May 1997; Tallahassee, USA. US Animal Health Association;p. 379–383.22. Nili H, Asasi K. Natural cases and an experimental study of H9N2 avian influenza in commercial broiler chickens of Iran. Avian Pathol. 2002; 31:247–252.
Article23. North MO, Bell DD. Commercial Chicken Production Manual. 4th ed. New York: Springer US;1990. p. 433–452.24. Skeeles JK, Beasley JN, Blore P, Klopp S. Severe egg-production drops in turkey breeders in southcentral Missouri. Avian Dis. 1981; 25:764–767.
Article25. Smyth JA, Platten MA, McFerran JB. A study of the pathogenesis of egg drop syndrome in laying hens. Avian Pathol. 1988; 17:653–666.
Article26. Swayne DE, Beck JR, Perdue ML, Beard CW. Efficacy of vaccines in chickens against highly pathogenic Hong Kong H5N1 avian influenza. Avian Dis. 2001; 45:355–365.
Article27. Swayne DE, Garcia M, Beck JR, Kinney N, Suarez DL. Protection against diverse highly pathogenic H5 avian influenza viruses in chickens immunized with a recombinant fowlpox vaccine containing an H5 avian influenza hemagglutinin gene insert. Vaccine. 2000; 18:1088–1095.
Article28. Tumpey TM, Kapczynski DR, Swayne DE. Comparative susceptibility of chickens and turkeys to avian influenza A H7N2 virus infection and protective efficacy of a commercial avian influenza H7N2 virus vaccine. Avian Dis. 2004; 48:167–176.
Article29. Van Eck JH, Davelaar FG, Van Den Heuvel-Plesman TAM, Van Kol N, Kouwenhoven B, Guldie FHM. Dropped egg production, soft shelled and shell-less eggs associated with appearance of precipitins to adenovirus in flocks of laying fowls. Avian Pathol. 1976; 5:261–272.
Article30. Williams JE, Dillard LH. Penetration patterns of Mycoplasma gallisepticum and Newcastle disease virus through the outer structures of chicken eggs. Avian Dis. 1968; 12:650–657.
Article31. Zanella A, Dall'Ara P, Martino PA. Avian influenza epidemic in Italy due to serovar H7N1. Avian Dis. 2001; 45:257–261.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Molecular identification of the vaccine strain from the inactivated oil emulsion H9N2 low pathogenic avian influenza vaccine
- H9N2 avian influenza virus in Korea: evolution and vaccination
- An inactivated vaccine to control the current H9N2 low pathogenic avian influenza in Korea
- Current situation and control strategies of H9N2 avian influenza in South Korea
- H5 and H9 subtypes of Avian Influenza Viruses are Real Threat To Humans