Lab Anim Res.
2016 Dec;32(4):257-266. 10.5625/lar.2016.32.4.257.
Establishment of a surgically induced cryptorchidism canine recipient model for spermatogonial stem cell transplantation
- Affiliations
-
- 1Department of Food Bioscience, RIBHS, College of Biomedical & Health Science, Konkuk University, Chungju, Korea.
- 2Department of Stem Cell and Regenerative Biology, Konkuk University, Seoul, Korea.
- 3Animal Biotechnology Division, National Institute of Animal Science, RDA, Jeonju, Korea.
- 4Metabolism and Nutrition Research Group, Korea Food Research Institute, Seongnam, Korea.
- 5Research Group of Nutraceuticals for Metabolic Syndrome, Korea Food Research Institute, Seongnam, Korea. vetian@kfri.re.kr
- KMID: 2362918
- DOI: http://doi.org/10.5625/lar.2016.32.4.257
Abstract
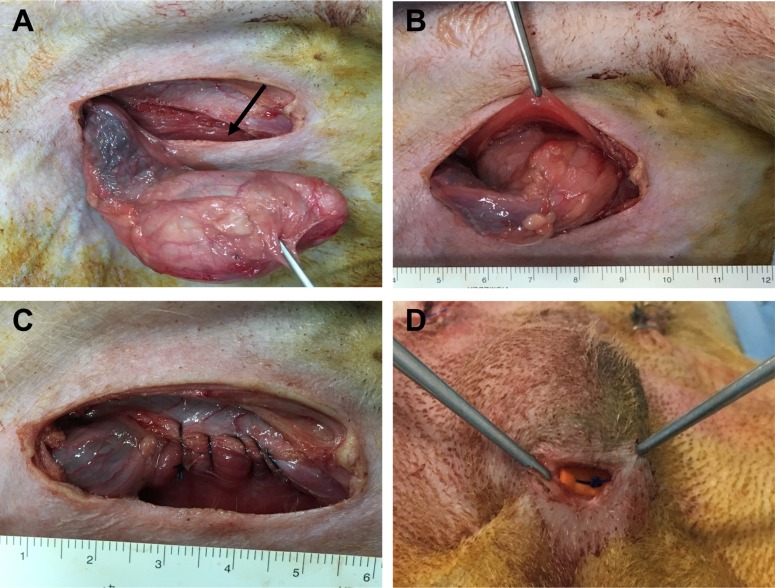
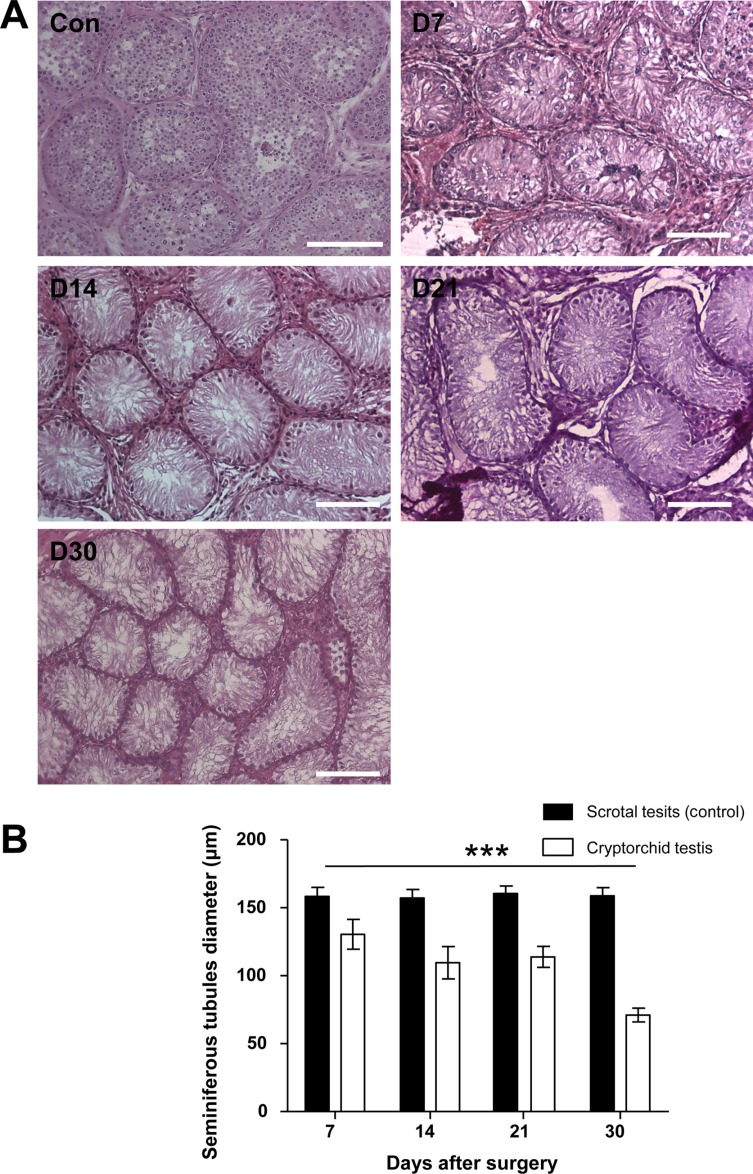
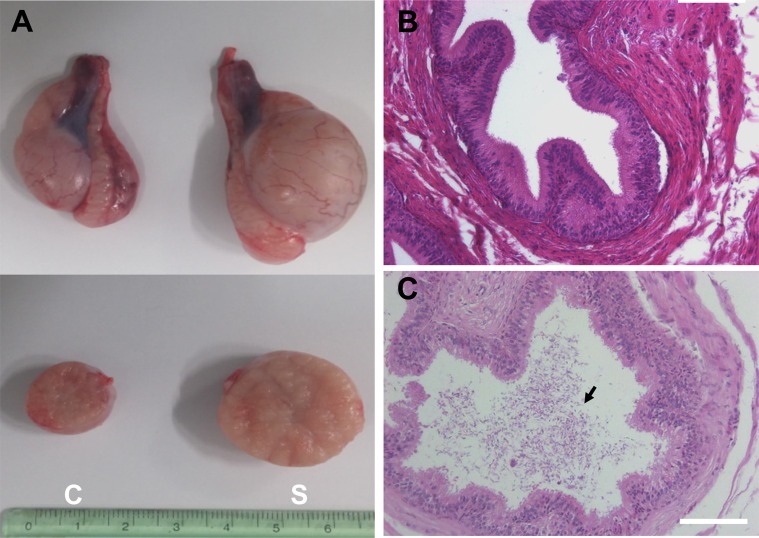
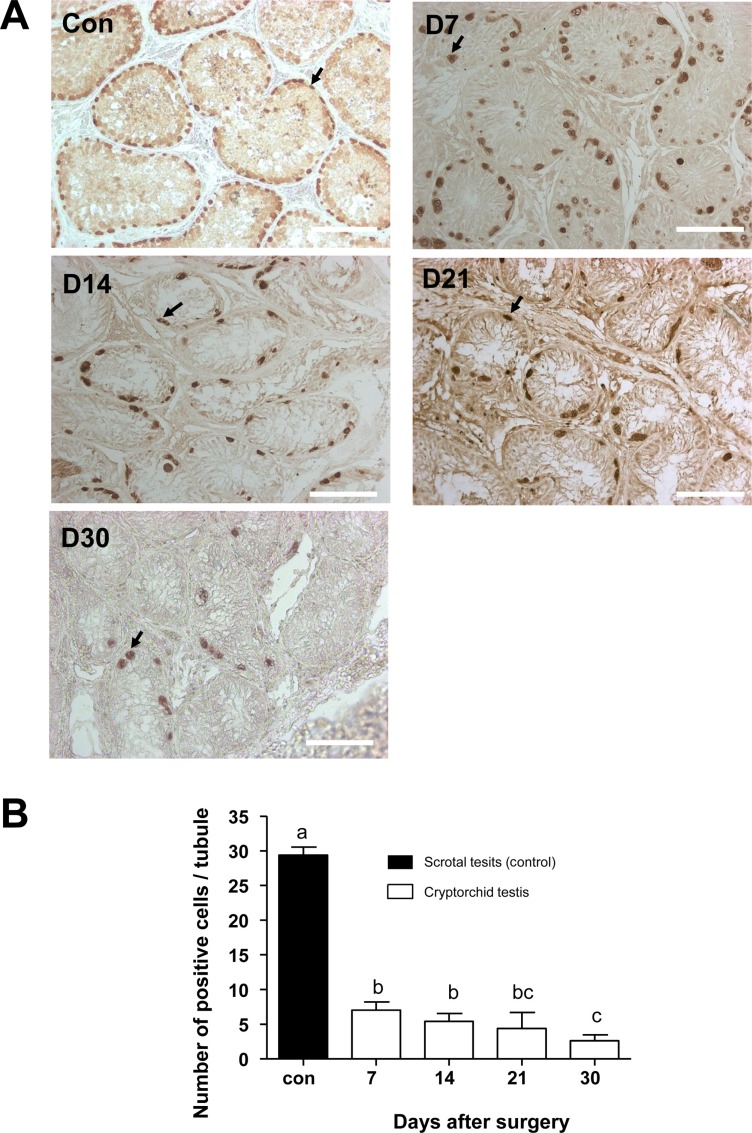
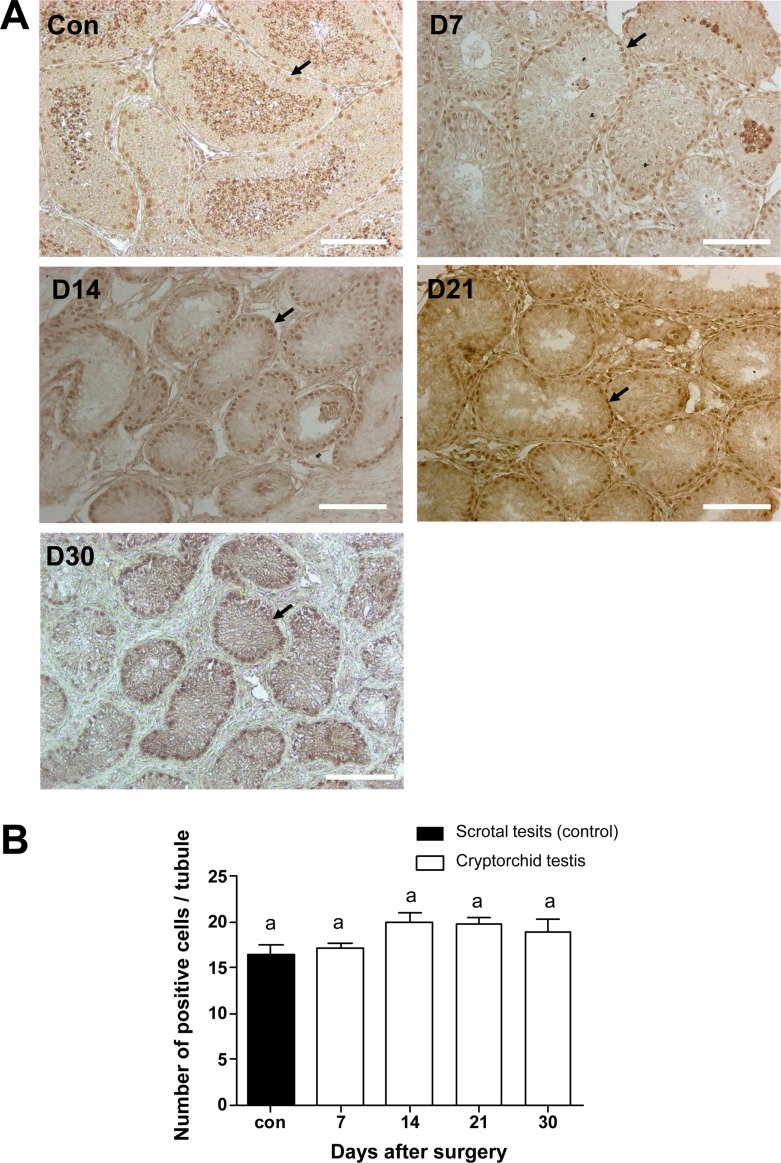
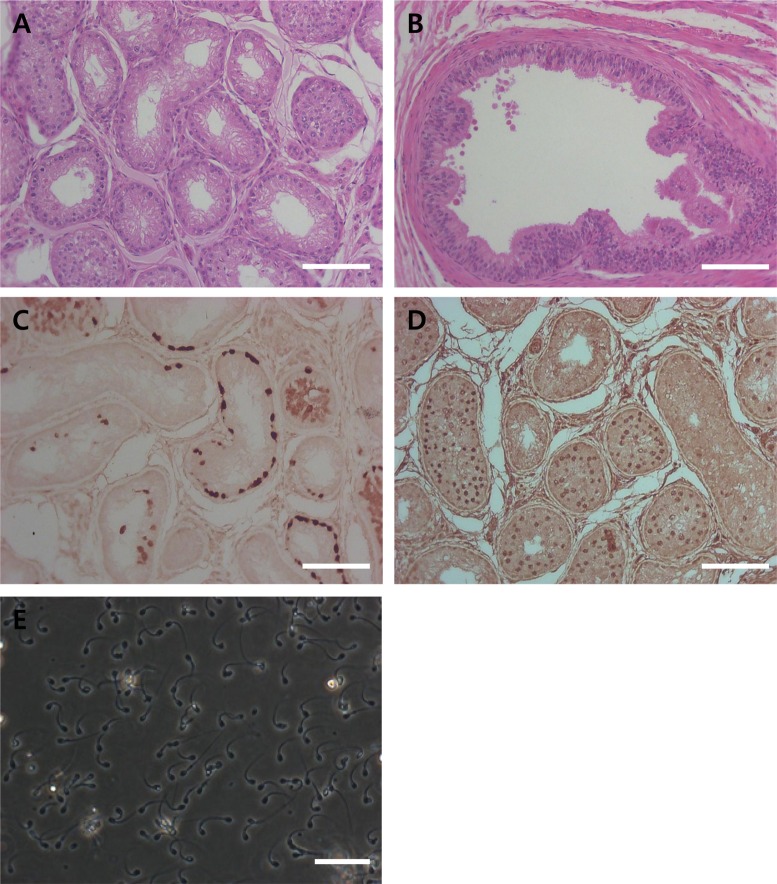
- Transplantation of spermatogonial stem cells (SSCs) in experimental animal models has been used to study germ line stem cell biology and to produce transgenic animals. The species-specific recipient model preparation is important for the characterization of SSCs and the production of offspring. Here, we investigated the effects of surgically induced cryptorchidism in dog as a new recipient model for spermatogonial stem cell transplantation. Artificially unilateral or bilateral cryptorchidism was induced in ten mature male dogs by surgically returning the testis and epididymis to the abdominal cavity. The testes and epididymides were collected every week after the induction of artificial cryptorchidism (surgery) for one month. To determine the effect of surgical cryptorchidism, the seminiferous tubule diameter was measured and immunohistochemistry using PGP9.5 and GATA4 antibodies was analyzed. The diameters of the seminiferous tubules of abdominal testes were significantly reduced compared to those of the scrotal testes. Immunohistochemistry results showed that PGP9.5 positive undifferentiated spermatogonia were significantly reduced after surgical cryptorchidism induction, but there were no significant changes in GATA-4 positive sertoli cells. To evaluate the testis function recovery rate, orchiopexy was performed on two dogs after 30 days of bilateral cryptorchidism. In the orchiopexy group, SCP3 positive spermatocytes were detected, and spermatogenesis was recovered 8 weeks after orchiopexy. In this study, we provided optimum experimental conditions and time for surgical preparation of a recipient canine model for SSC transplantation. Additionally, our data will contribute to recipient preparation by using surgically induced cryptorchidism in non-rodent species.
Keyword
MeSH Terms
-
Abdominal Cavity
Animals
Animals, Genetically Modified
Antibodies
Biology
Cryptorchidism*
Dogs
Epididymis
Germ Cells
Humans
Immunohistochemistry
Male
Models, Animal
Orchiopexy
Recovery of Function
Seminiferous Tubules
Sertoli Cells
Spermatocytes
Spermatogenesis
Spermatogonia
Stem Cell Transplantation*
Stem Cells*
Testis
Antibodies
Figure
Reference
-
1. de Rooij DG, Grootegoed JA. Spermatogonial stem cells. Curr Opin Cell Biol. 1998; 10(6):694–701. PMID: 9914171.
Article2. Brinster RL, Zimmermann JW. Spermatogenesis following male germ-cell transplantation. Proc Natl Acad Sci U S A. 1994; 91(24):11298–11302. PMID: 7972053.
Article3. Kim Y, Turner D, Nelson J, Dobrinski I, McEntee M, Travis AJ. Production of donor-derived sperm after spermatogonial stem cell transplantation in the dog. Reproduction. 2008; 136(6):823–831. PMID: 18768666.
Article4. Kim Y, Selvaraj V, Dobrinski I, Lee H, McEntee MC, Travis AJ. Recipient preparation and mixed germ cell isolation for spermatogonial stem cell transplantation in domestic cats. J Androl. 2006; 27(2):248–256. PMID: 16304210.
Article5. Honaramooz A, Behboodi E, Blash S, Megee SO, Dobrinski I. Germ cell transplantation in goats. Mol Reprod Dev. 2003; 64(4):422–428. PMID: 12589654.
Article6. Izadyar F, Den Ouden K, Stout TA, Stout J, Coret J, Lankveld DP, Spoormakers TJ, Colenbrander B, Oldenbroek JK, Van der Ploeg KD, Woelders H, Kal HB, De Rooij DG. Autologous and homologous transplantation of bovine spermatogonial stem cells. Reproduction. 2003; 126(6):765–774. PMID: 14748695.
Article7. Honaramooz A, Megee SO, Dobrinski I. Germ cell transplantation in pigs. Biol Reprod. 2002; 66(1):21–28. PMID: 11751259.8. Brinster RL. Germline stem cell transplantation and transgenesis. Science. 2002; 296(5576):2174–2176. PMID: 12077400.
Article9. Ogawa T, Aréchaga JM, Avarbock MR, Brinster RL. Transplantation of testis germinal cells into mouse seminiferous tubules. Int J Dev Biol. 1997; 41(1):111–122. PMID: 9074943.10. Brinster CJ, Ryu BY, Avarbock MR, Karagenc L, Brinster RL, Orwig KE. Restoration of fertility by germ cell transplantation requires effective recipient preparation. Biol Reprod. 2003; 69(2):412–420. PMID: 12672656.11. Ogawa T, Dobrinski I, Brinster RL. Recipient preparation is critical for spermatogonial transplantation in the rat. Tissue Cell. 1999; 31(5):461–472. PMID: 10612257.
Article12. Honaramooz A, Behboodi E, Hausler CL, Blash S, Ayres S, Azuma C, Echelard Y, Dobrinski I. Depletion of endogenous germ cells in male pigs and goats in preparation for germ cell transplantation. J Androl. 2005; 26(6):698–705. PMID: 16291964.
Article13. Jahnukainen K, Ehmcke J, Quader MA, Saiful Huq M, Epperly MW, Hergenrother S, Nurmio M, Schlatt S. Testicular recovery after irradiation differs in prepubertal and pubertal non-human primates, and can be enhanced by autologous germ cell transplantation. Hum Reprod. 2011; 26(8):1945–1954. PMID: 21613315.
Article14. Zhang Z, Shao S, Meistrich ML. Irradiated mouse testes efficiently support spermatogenesis derived from donor germ cells of mice and rats. J Androl. 2006; 27(3):365–375. PMID: 16339450.
Article15. Griffiths J. The Structural Changes in the Testicle of the Dog when it is Replaced within the Abdominal Cavity. J Anat Physiol. 1893; 27(Pt 4):482–500.16. Shirai M, Matsushita S, Kagayama M, Ichijo S, Takeuchi M. Histological changes of the scrotal testis in unilateral cryptorchidism. Tohoku J Exp Med. 1966; 90(4):363–373. PMID: 4382089.
Article17. Hall PF, Kew D, Mita M. The influence of temperature on the functions of cultured Sertoli cells. Endocrinology. 1985; 116(5):1926–1932. PMID: 2985365.
Article18. Hill JR, Dobrinski I. Male germ cell transplantation in livestock. Reprod Fertil Dev. 2006; 18(1-2):13–18. PMID: 16478598.
Article19. Deeg HJ, Schuler US, Shulman H, Ehrsam M, Renner U, Yu C, Storb R, Ehninger G. Myeloablation by intravenous busulfan and hematopoietic reconstitution with autologous marrow in a canine model. Biol Blood Marrow Transplant. 1999; 5(5):316–321. PMID: 10534062.
Article20. Monet-Kuntz C, Barenton B, Locatelli A, Fontaine I, Perreau C, Hochereau-de Reviers MT. Effects of experimental cryptorchidism and subsequent orchidopexy on seminiferous tubule functions in the lamb. J Androl. 1987; 8(3):148–154. PMID: 2886484.21. Amat P, Paniagua R, Montero J. Seminiferous tubule degeneration in human cryptorchid testes. J Androl. 1985; 6(1):1–9. PMID: 2857707.
Article22. AbouZeid AA, Mousa MH, Soliman HA, Hamza AF, Hay SA. Intra-abdominal testis: histological alterations and significance of biopsy. J Urol. 2011; 185(1):269–274. PMID: 21075394.
Article23. Sutovsky P. Ubiquitin-dependent proteolysis in mammalian spermatogenesis, fertilization, and sperm quality control: killing three birds with one stone. Microsc Res Tech. 2003; 61(1):88–102. PMID: 12672125.
Article24. Harkey MA, Asano A, Zoulas ME, Torok-Storb B, Nagashima J, Travis A. Isolation, genetic manipulation, and transplantation of canine spermatogonial stem cells: progress toward transgenesis through the male germ-line. Reproduction. 2013; 146(1):75–90. PMID: 23690628.
Article25. Viger RS, Guittot SM, Anttonen M, Wilson DB, Heikinheimo M. Role of the GATA family of transcription factors in endocrine development, function, and disease. Mol Endocrinol. 2008; 22(4):781–798. PMID: 18174356.
Article26. Bielinska M, Seehra A, Toppari J, Heikinheimo M, Wilson DB. GATA-4 is required for sex steroidogenic cell development in the fetal mouse. Dev Dyn. 2007; 236(1):203–213. PMID: 17096405.
Article27. Ketola I, Rahman N, Toppari J, Bielinska M, Porter-Tinge SB, Tapanainen JS, Huhtaniemi IT, Wilson DB, Heikinheimo M. Expression and regulation of transcription factors GATA-4 and GATA-6 in developing mouse testis. Endocrinology. 1999; 140(3):1470–1480. PMID: 10067876.28. Jegou B, Peake RA, Irby DC, de Kretser DM. Effects of the induction of experimental cryptorchidism and subsequent orchidopexy on testicular function in immature rats. Biol Reprod. 1984; 30(1):179–187. PMID: 6141812.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Opening the era of in vivo xenotransplantation model for hematopoietic stem cell transplantation
- Dose-dependent effects of busulfan on dog testes in preparation for spermatogonial stem cell transplantation
- Safety and outcomes of subconjunctival allogenic mesenchymal stem cell transplantation in canine experimental corneal defects
- Inhibition of Class I Histone Deacetylase Enhances Self-Reprogramming of Spermatogonial Stem Cells into Pluripotent Stem Cells
- Stem Cell Therapy for Type 1 Diabetes Mellitus