Lab Anim Res.
2016 Dec;32(4):181-186. 10.5625/lar.2016.32.4.181.
Evaluation of stability and biocompatibility of PHEMA-PMMA keratoprosthesis by penetrating keratoplasty in rabbits
- Affiliations
-
- 1Department of Veterinary Surgery, College of Veterinary Medicine, Chungbuk National University, Cheongju, Korea. ghkim@cbu.ac.kr
- KMID: 2362908
- DOI: http://doi.org/10.5625/lar.2016.32.4.181
Abstract
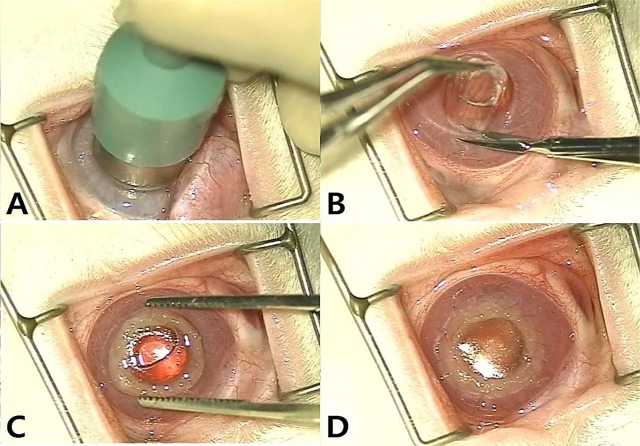
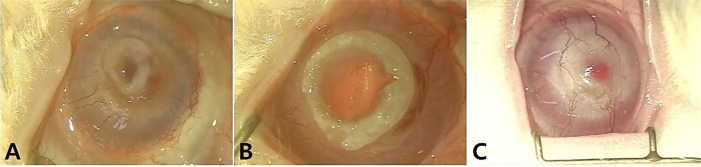
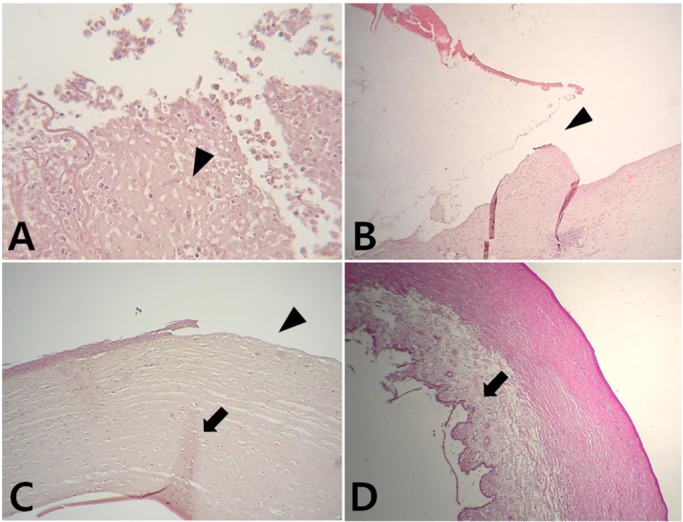
- Artificial corneas have been developed as an alternative to natural donor tissue to replace damaged or diseased corneas. This study was conducted to evaluate the stability and biocompatibility of PHEMA-PMMA [poly (2-hydroxyl methacrylate)-poly (methyl methacrylate)] keratoprostheses in rabbits following penetrating keratoplasty. Sixteen male New Zealand White rabbits aged 16 weeks were divided into three groups. Group I and group II contained six rabbits each, while the control group had four rabbits. Experimental surgery was conducted under general anesthesia. The cornea was penetrated using an 8 mm diameter biopsy punch. In group I (core 5 mm & skirt 3 mm) and group II (core 6 mm & skirt 2 mm), the keratoprosthesis was placed into the recipient full thickness bed and sutured into position with double-layer continuous. In the control group, corneal transplantation using normal allogenic corneal tissue was performed with the same suture method. After four and eight weeks, keratoprosthesis devices were evaluated by histopathological analysis of gross lesions. Post-operative complications were observed, such as extrusion and infection in experimental groups. Most corneas were maintained in the defect site by double-layer continuous suture materials for 4 weeks and kept good light transmission. However, most artificial cornea were extruded before 8 weeks. Overall, combined PHEMA and PMMA appears to have sufficient advantages for production of artificial corneas because of its optical transparency, flexibility and other mechanical features. However, the stability and biocompatibility were not sufficient to enable application in humans and animals at the present time using penetrating keratoplasty. Further studies are essential to improve the stability and biocompatibility with or without other types of keratoplasty.
Keyword
MeSH Terms
Figure
Reference
-
1. Bleckmann H, Holak S. Preliminary results after implantation of four AlphaCor artificial corneas. Graefes Arch Clin Exp Ophthalmol. 2006; 244(4):502–506. PMID: 16133028.
Article2. Chirila TV. An overview of the development of artificial corneas with porous skirts and the use of PHEMA for such an application. Biomaterials. 2001; 22(24):3311–3317. PMID: 11700803.
Article3. Cuperus PL, Jongebloed WL, van Andel P, Worst JG. Glass-metal keratoprosthesis: light and electron microscopical evaluation of experimental surgery on rabbit eyes. Doc Ophthalmol. 1989; 71(1):29–47. PMID: 2663392.
Article4. Guo P, Chen JQ, Huang LN, Wang Z, Wang ZC, Nie DY. Implantation of modified poly 2-hydroxyethy methacrylate-Polymethyl methacrylate Keratoprosthesis in rabbit and monkey corneas. Int J Ophthalmol. 2009; 2(4):310–315.5. Hicks CR, Chirila TV, Clayton AB, Fitton JH, Vijayasekaran S, Dalton PD, Lou X, Platten S, Ziegelaar B, Hong Y, Crawford GJ, Constable IJ. Clinical results of implantation of the Chirila keratoprosthesis in rabbits. Br J Ophthalmol. 1998; 82(1):18–25. PMID: 9536874.
Article6. Hicks CR, Fitton JH, Chirila TV, Crawford GJ, Constable IJ. Keratoprostheses: advancing toward a true artificial cornea. Surv Ophthalmol. 1997; 42(2):175–189. PMID: 9381372.
Article7. Isard PF, Dulaurent T, Regnier A. Keratoprosthesis with retrocorneal fixation: preliminary results in dogs with corneal blindness. Vet Ophthalmol. 2010; 13(5):279–288. PMID: 20840104.
Article8. Jiraskova N, Werner L, Mamalis N, Rozsival P. Histologic evaluation of AlphaCor keratoprosthesis explanted following various complications. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2014; 158(1):50–55. PMID: 23073521.
Article9. Khan B, Dudenhoefer EJ, Dohlman CH. Keratoprosthesis: an update. Curr Opin Ophthalmol. 2001; 12(4):282–287. PMID: 11507341.
Article10. Lee JH, Wee WR, Chung ES, Kim HY, Park SH, Kim YH. Development of a newly designed double-fixed Seoul-type keratoprosthesis. Arch Ophthalmol. 2000; 118(12):1673–1678. PMID: 11115262.
Article11. Legeais JM, Rossi C, Renard G, Salvoldelli M, D'Hermies F, Pouliquen YJ. A new fluorocarbon for keratoprosthesis. Cornea. 1992; 11(6):538–545. PMID: 1468216.
Article12. Liu Y, Gan L, Carlsson DJ, Fagerholm P, Lagali N, Watsky MA, Munger R, Hodge WG, Priest D, Griffith M. A simple, cross-linked collagen tissue substitute for corneal implantation. Invest Ophthalmol Vis Sci. 2006; 47(5):1869–1875. PMID: 16638993.
Article13. Lou X, Dalton PD, Chirila TV. Hydrophilic sponges based on 2-hydroxyethyl methacrylate: part VII: modulation of sponge characteristics by changes in reactivity and hydrophilicity of crosslinking agents. J Mater Sci Mater Med. 2000; 11(5):319–325. PMID: 15348030.14. Moffatt SL, Cartwright VA, Stumpf TH. Centennial review of corneal transplantation. Clin Exp Ophthalmol. 2005; 33(6):642–657. PMID: 16402960.
Article15. Myung D, Duhamel PE, Cochran JR, Noolandi J, Ta CN, Frank CW. Development of hydrogel-based keratoprostheses: a materials perspective. Biotechnol Prog. 2008; 24(3):735–741. PMID: 18422366.
Article16. Nardi M, Giudice V, Marabotti A, Alfieri E, Rizzo S. Temporary graft for closed-system cataract surgery during corneal triple procedures. J Cataract Refract Surg. 2001; 27(8):1172–1175. PMID: 11524186.
Article17. Peppas NA, Huang Y, Torres-Lugo M, Ward JH, Zhang J. Physicochemical foundations and structural design of hydrogels in medicine and biology. Annu Rev Biomed Eng. 2000; 2:9–29. PMID: 11701505.
Article18. Pereira FQ, Bercht BS, Soares MG, da Mota MG, Pigatto JA. Comparison of a rebound and an applanation tonometer for measuring intraocular pressure in normal rabbits. Vet Ophthalmol. 2011; 14(5):321–326. PMID: 21929609.
Article19. Sandeman SR, Lloyd AW, Tighe BJ, Franklin V, Li J, Lydon F, Liu CS, Mann DJ, James SE, Martin R. A model for the preliminary biological screening of potential keratoprosthetic biomaterials. Biomaterials. 2003; 24(26):4729–4739. PMID: 14530070.
Article20. Vajpayee RB, Sharma V, Sharma N, Panda A, Taylor HR. Evaluation of techniques of single continuous suturing in penetrating keratoplasty. Br J Ophthalmol. 2001; 85(2):134–138. PMID: 11159473.
Article21. Wang L, Jeong KJ, Chiang HH, Zurakowski D, Behlau I, Chodosh J, Dohlman CH, Langer R, Kohane DS. Hydroxyapatite for keratoprosthesis biointegration. Invest Ophthalmol Vis Sci. 2011; 52(10):7392–7399. PMID: 21849419.
Article22. Whitcher JP, Srinivasan M, Upadhyay MP. Corneal blindness: a global perspective. Bull World Health Organ. 2001; 79(3):214–221. PMID: 11285665.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A tectonic keratoprosthesis using expanded polytetrafluoroethylene as a supporting skirt in humans
- Clinical, Physical Stability and Histological Biocompatibility of Experimental Seoul Type Keratoprosthesis
- Animal Experiment for Comparison of Seoul Type Keratoprosthesis with Improved Biocompatibility and Conventional Keratoprosthesis
- The experimental Seoul-type keratoprosthesis
- The Effect of Surface Modification on Retention Period of Seoul Type Keratoprosthesis