J Pathol Transl Med.
2016 Nov;50(6):436-441. 10.4132/jptm.2016.07.12.
Comparison of the FDA and ASCO/CAP Criteria for HER2 Immunohistochemistry in Upper Urinary Tract Urothelial Carcinoma
- Affiliations
-
- 1Department of Pathology, Medical Research Center, Seoul National University College of Medicine, Seoul, Korea. blue7270@snu.ac.kr
- 2Kidney Research Institute, Medical Research Center, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2361991
- DOI: http://doi.org/10.4132/jptm.2016.07.12
Abstract
- BACKGROUND
Human epidermal growth factor receptor 2 (HER2) is one of the known oncogenes in urothelial carcinoma. However, the association between HER2 and the prognosis of upper urinary tract urothelial carcinoma (UUTUC) has not yet been fully clarified. The aim of this study was to evaluate HER2 expression using the United States Food and Drug Administration (FDA) criteria and American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) criteria and compare their prognostic significance in UUTUC.
METHODS
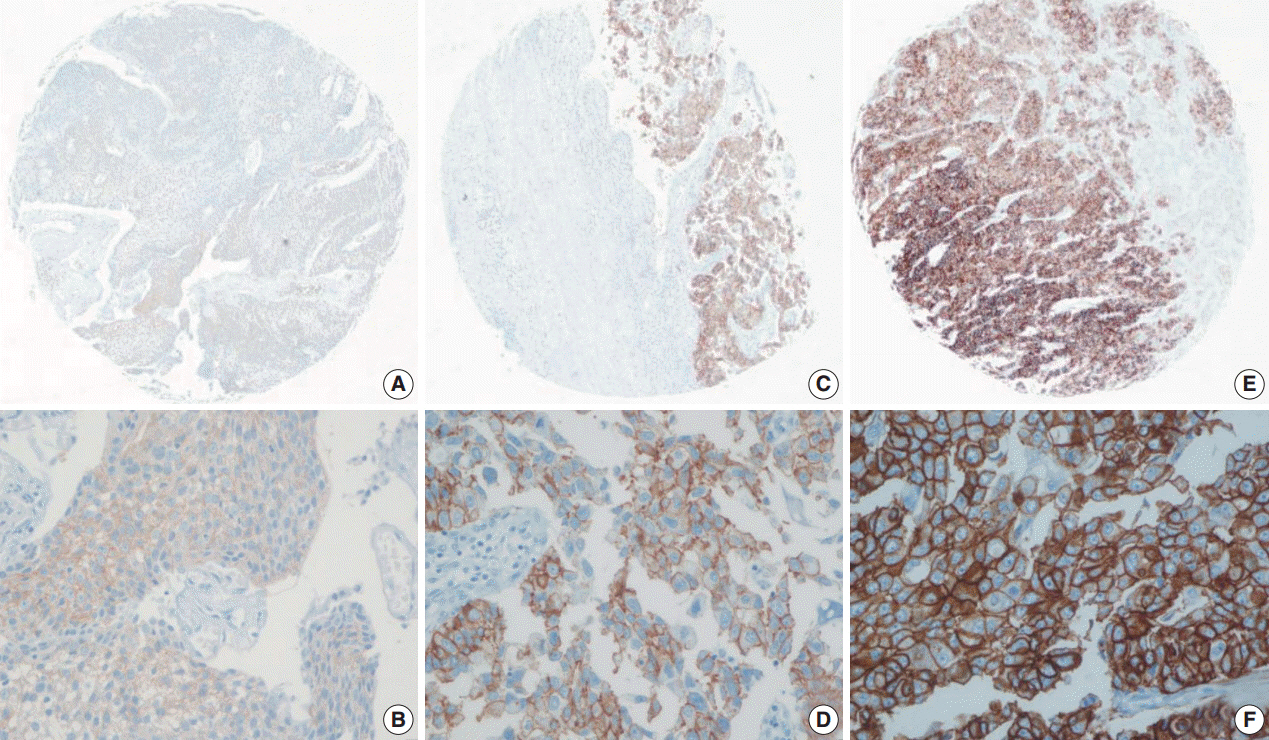
HER2 expression was evaluated in 144 cases of UUTUC by immunohistochemistry (IHC) using tissue microarrays. We separately analyzed HER2 expression using the FDA and ASCO/CAP criteria. The IHC results were categorized into low (0, 1+) and high (2+, 3+) groups.
RESULTS
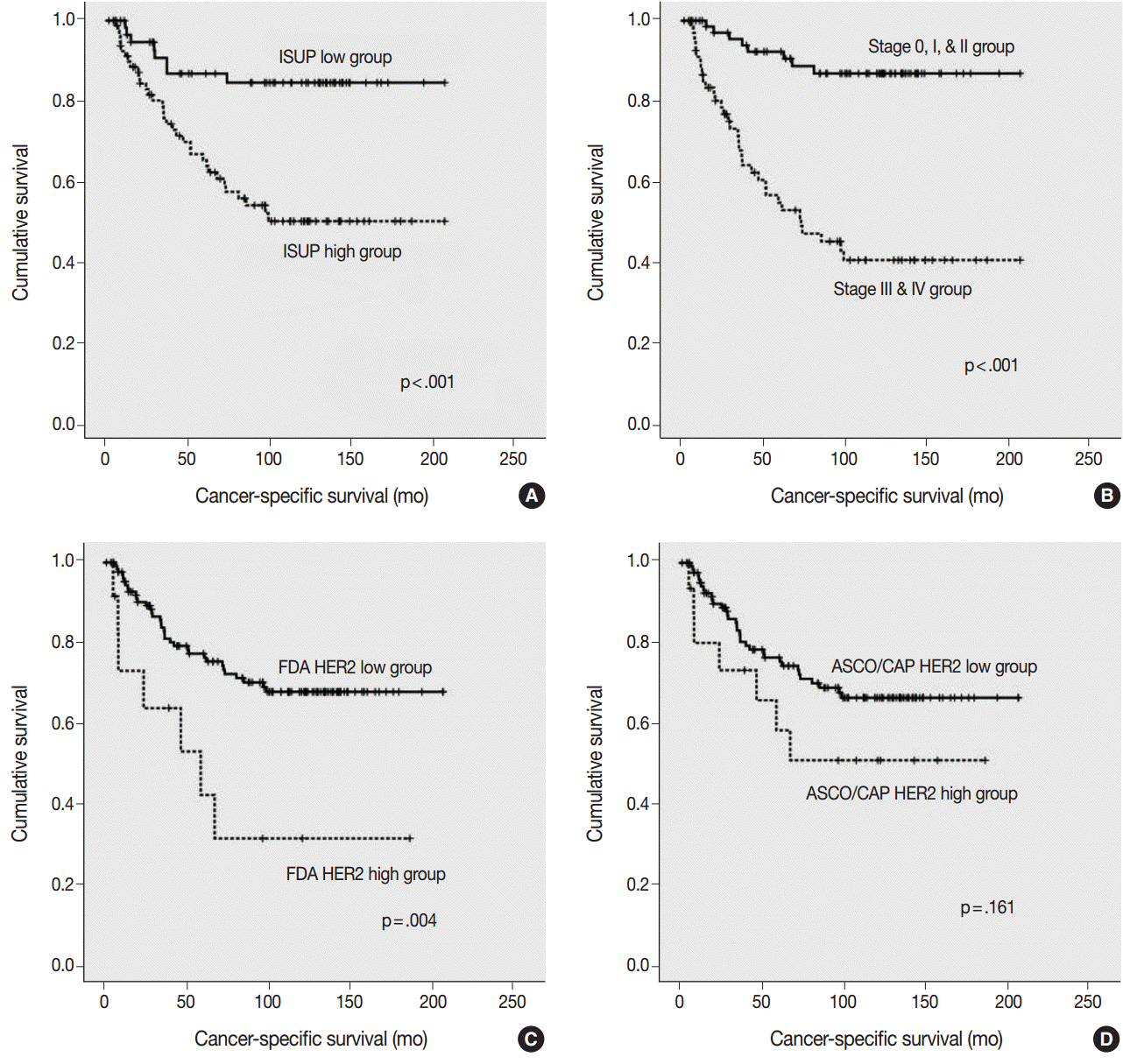
Using the FDA criteria, 94 cases were negative, 38 cases were 1+, nine cases were 2+, and three cases were 3+. Using the ASCO/CAP criteria, 94 cases were negative, 34 cases were 1+, 13 cases were 2+, and three cases were 3+. Four cases showing 2+ according to the ASCO/CAP criteria were reclassified as 1+ by the FDA criteria. High HER2 expression by both the FDA criteria and ASCO/CAP criteria was significantly associated with International Society of Urological Pathology high grade (p = .001 and p < .001). The high HER2 expression group classified with the FDA criteria showed significantly shorter cancer-specific survival (p = .004), but the HER2 high and low expression groups classified with the ASCO/CAP criteria did not show significant differences (p = .161) in cancer-specific survival.
CONCLUSIONS
HER2 high expression groups were significantly associated with shorter cancer-specific survival, and our study revealed that the FDA criteria are more suitable for determining HER2 expression in UUTUC.
MeSH Terms
Figure
Reference
-
1. Langner C, Gross C, Rehak P, Ratschek M, Ruschoff J, Zigeuner R. HER2 protein overexpression and gene amplification in upper urinary tract transitional cell carcinoma: systematic analysis applying tissue microarray technique. Urology. 2005; 65:176–80.
Article2. Vershasselt-Crinquette M, Colin P, Ouzzane A, et al. Assessment of human epidermal growth factor receptor 2 status in urothelial carcinoma of the upper urinary tract: a study using dual-color in situ hybridization and immunohistochemistry. Appl Immunohistochem Mol Morphol. 2012; 20:363–6.3. Tsai YS, Tzai TS, Chow NH, Wu CL. Frequency and clinicopathologic correlates of ErbB1, ErbB2, and ErbB3 immunoreactivity in urothelial tumors of upper urinary tract. Urology. 2005; 66:1197–202.
Article4. Olayioye MA, Neve RM, Lane HA, Hynes NE. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J. 2000; 19:3159–67.5. Tinoco G, Warsch S, Gluck S, Avancha K, Montero AJ. Treating breast cancer in the 21st century: emerging biological therapies. J Cancer. 2013; 4:117–32.
Article6. Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010; 28:2784–95.
Article7. Smyth EC, Cunningham D. Targeted therapy for gastric cancer. Curr Treat Options Oncol. 2012; 13:377–89.
Article8. Bang YJ. Advances in the management of HER2-positive advanced gastric and gastroesophageal junction cancer. J Clin Gastroenterol. 2012; 46:637–48.
Article9. Kunz PL, Mojtahed A, Fisher GA, et al. HER2 expression in gastric and gastroesophageal junction adenocarcinoma in a US population: clinicopathologic analysis with proposed approach to HER2 assessment. Appl Immunohistochem Mol Morphol. 2012; 20:13–24.10. Chibon F, de Mascarel I, Sierankowski G, et al. Prediction of HER2 gene status in Her2 2+ invasive breast cancer: a study of 108 cases comparing ASCO/CAP and FDA recommendations. Mod Pathol. 2009; 22:403–9.
Article11. Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001; 344:783–92.
Article12. Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013; 31:3997–4013.13. Sasaki Y, Sasaki T, Kawai T, et al. HER2 protein overexpression and gene amplification in upper urinary tract urothelial carcinoma-an analysis of 171 patients. Int J Clin Exp Pathol. 2014; 7:699–708.14. Lae M, Couturier J, Oudard S, Radvanyi F, Beuzeboc P, Vieillefond A. Assessing HER2 gene amplification as a potential target for therapy in invasive urothelial bladder cancer with a standardized methodology: results in 1005 patients. Ann Oncol. 2010; 21:815–9.15. Hansel DE, Swain E, Dreicer R, Tubbs RR. HER2 overexpression and amplification in urothelial carcinoma of the bladder is associated with MYC coamplification in a subset of cases. Am J Clin Pathol. 2008; 130:274–81.16. Krüger S, Weitsch G, Büttner H, et al. HER2 overexpression in muscle-invasive urothelial carcinoma of the bladder: prognostic implications. Int J Cancer. 2002; 102:514–8.
Article17. Ruschoff J, Hanna W, Bilous M, et al. HER2 testing in gastric cancer: a practical approach. Mod Pathol. 2012; 25:637–50.
Article18. Buza N, English DP, Santin AD, Hui P. Toward standard HER2 testing of endometrial serous carcinoma: 4-year experience at a large academic center and recommendations for clinical practice. Mod Pathol. 2013; 26:1605–12.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- HER2 status in breast cancer: changes in guidelines and complicating factors for interpretation
- Automated Silver-enhanced In Situ Hybridization for Evaluation of HER2 Gene Status in Breast Carcinoma: Comparison with Fluorescence In Situ Hybridization and Immunohistochemistry
- Urothelial Tumors of the Upper Urinary Tract: Multiplicity and Prognostic Variables
- Urothelial Tumors of the Upper Urinary Tract, 15 Cases
- A clinical analysis of the upper urinary tract transitional cell carcinoma and associated bladder tumor