J Korean Soc Radiol.
2016 Dec;75(6):434-445. 10.3348/jksr.2016.75.6.434.
Change in Functional Connectivity in Tinnitus and its Relation with Tinnitus Laterality
- Affiliations
-
- 1Department of Radiology, College of Medicine, Kyung Hee University, Seoul, Korea.
- 2Department of Radiology, Kyung Hee University Hospital at Gangdong, School of Medicine, Kyung Hee University, Seoul, Korea. md.cwryu@gmail.com
- 3Department of Otorhinolaryngology, Kyung Hee University Hospital at Gangdong, School of Medicine, Kyung Hee University, Seoul, Korea.
- KMID: 2360430
- DOI: http://doi.org/10.3348/jksr.2016.75.6.434
Abstract
- PURPOSE
To identify potential differences in resting-state networks according to laterality of tinnitus using resting-state functional MRI (fMRI).
MATERIALS AND METHODS
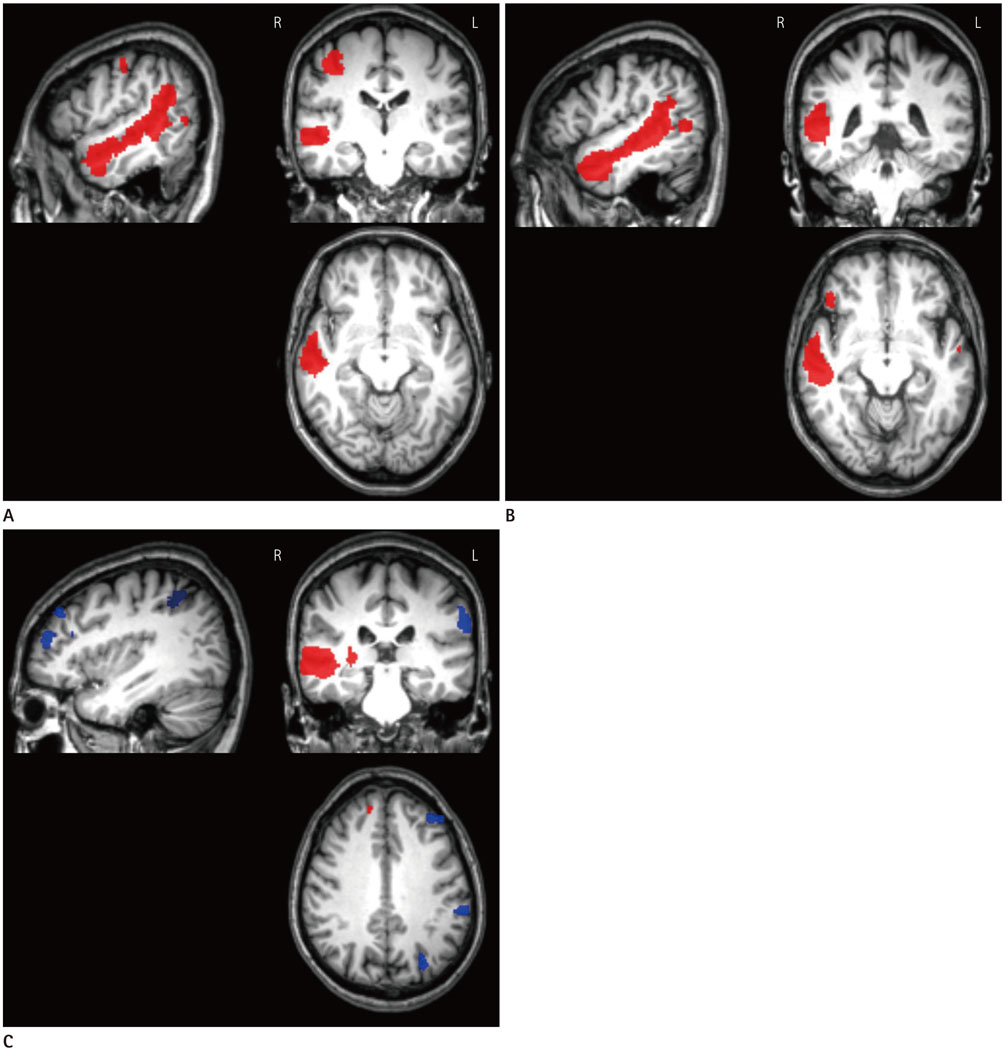
A total of 83 age-matched subjects consisting of 19 patients with right-sided tinnitus (Rt-T), 22 patients with left-sided tinnitus (Lt-T), 22 patients with bilateral tinnitus (Bil-T), and 20 healthy controls underwent resting-state blood oxygenation-level dependent fMRI scans. Independent component analysis was used to obtain the functional connectivities in the auditory network (AN) and the default mode network (DMN), which were compared between each group using the voxel-wise one-way ANOVA. In addition, lateralization of the auditory cortex was assessed within each group using a region of interest (ROI).
RESULTS
Comparisons between tinnitus groups showed unusual clusters with different functional connectivities in the AN and the DMN. The Rt-T group had large clusters with higher functional connectivity in the right middle temporal gyrus and temporopolar area compared with the Lt-/Bil-T and control groups. ROI analysis showed that the Rt-/Lt-T groups had dominant functional connectivity in the right auditory cortex and the Bil-T and control groups had left-dominant auditory connectivity.
CONCLUSION
These results suggest that chronic tinnitus is related to aberrant laterality of the auditory cortex. These findings help clarify the neural mechanism of tinnitus and specify the targets for localization of treatment.
MeSH Terms
Figure
Reference
-
1. Eggermont JJ, Roberts LE. The neuroscience of tinnitus. Trends Neurosci. 2004; 27:676–682.2. Han BI, Lee HW, Kim TY, Lim JS, Shin KS. Tinnitus: characteristics, causes, mechanisms, and treatments. J Clin Neurol. 2009; 5:11–19.3. Adjamian P, Sereda M, Hall DA. The mechanisms of tinnitus: perspectives from human functional neuroimaging. Hear Res. 2009; 253:15–31.4. Schlee W, Mueller N, Hartmann T, Keil J, Lorenz I, Weisz N. Mapping cortical hubs in tinnitus. BMC Biol. 2009; 7:80.5. Schlee W, Weisz N, Bertrand O, Hartmann T, Elbert T. Using auditory steady state responses to outline the functional connectivity in the tinnitus brain. PLoS One. 2008; 3:e3720.6. Maudoux A, Lefebvre P, Cabay JE, Demertzi A, Vanhaudenhuyse A, Laureys S, et al. Auditory resting-state network connectivity in tinnitus: a functional MRI study. PLoS One. 2012; 7:e36222.7. Kim JY, Kim YH, Lee S, Seo JH, Song HJ, Cho JH, et al. Alteration of functional connectivity in tinnitus brain revealed by resting-state fMRI? A pilot study. Int J Audiol. 2012; 51:413–441.8. Davies J, Gander PE, Andrews M, Hall DA. Auditory network connectivity in tinnitus patients: a resting-state fMRI study. Int J Audiol. 2014; 53:192–198.9. Burton H, Wineland A, Bhattacharya M, Nicklaus J, Garcia KS, Piccirillo JF. Altered networks in bothersome tinnitus: a functional connectivity study. BMC Neurosci. 2012; 13:3.10. Ueyama T, Donishi T, Ukai S, Ikeda Y, Hotomi M, Yamanaka N, et al. Brain regions responsible for tinnitus distress and loudness: a resting-state FMRI study. PLoS One. 2013; 8:e67778.11. Maudoux A, Lefebvre P, Cabay JE, Demertzi A, Vanhaudenhuyse A, Laureys S, et al. Connectivity graph analysis of the auditory resting state network in tinnitus. Brain Res. 2012; 1485:10–21.12. Wineland AM, Burton H, Piccirillo J. Functional connectivity networks in nonbothersome tinnitus. Otolaryngol Head Neck Surg. 2012; 147:900–906.13. Schmidt SA, Akrofi K, Carpenter-Thompson JR, Husain FT. Default mode, dorsal attention and auditory resting state networks exhibit differential functional connectivity in tinnitus and hearing loss. PLoS One. 2013; 8:e76488.14. Lanting CP, de Kleine E, Langers DR, van Dijk P. Unilateral tinnitus: changes in connectivity and response lateralization measured with FMRI. PLoS One. 2014; 9:e110704.15. Boyen K, de Kleine E, van Dijk P, Langers DR. Tinnitus-related dissociation between cortical and subcortical neural activity in humans with mild to moderate sensorineural hearing loss. Hear Res. 2014; 312:48–59.16. Smits M, Kovacs S, de Ridder D, Peeters RR, van Hecke P, Sunaert S. Lateralization of functional magnetic resonance imaging (fMRI) activation in the auditory pathway of patients with lateralized tinnitus. Neuroradiology. 2007; 49:669–679.17. Arnold W, Bartenstein P, Oestreicher E, Römer W, Schwaiger M. Focal metabolic activation in the predominant left auditory cortex in patients suffering from tinnitus: a PET study with [18F]deoxyglucose. ORL J Otorhinolaryngol Relat Spec. 1996; 58:195–199.18. De Ridder D, De Mulder G, Verstraeten E, Van der Kelen K, Sunaert S, Smits M, et al. Primary and secondary auditory cortex stimulation for intractable tinnitus. ORL J Otorhinolaryngol Relat Spec. 2006; 68:48–54. discussion 54-55.19. van der Loo E, Congedo M, Vanneste S, Van De Heyning P, De Ridder D. Insular lateralization in tinnitus distress. Auton Neurosci. 2011; 165:191–194.20. Geven LI, de Kleine E, Willemsen AT, van Dijk P. Asymmetry in primary auditory cortex activity in tinnitus patients and controls. Neuroscience. 2014; 256:117–125.21. Calhoun VD, Adali T, Pearlson GD, Pekar JJ. A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp. 2001; 14:140–151.22. Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, et al. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A. 2006; 103:13848–13853.23. Chao LL, Haxby JV, Martin A. Attribute-based neural substrates in temporal cortex for perceiving and knowing about objects. Nat Neurosci. 1999; 2:913–919.24. Tranel D, Damasio H, Damasio AR. A neural basis for the retrieval of conceptual knowledge. Neuropsychologia. 1997; 35:1319–1327.25. Langguth B, Eichhammer P, Kreutzer A, Maenner P, Marienhagen J, Kleinjung T, et al. The impact of auditory cortex activity on characterizing and treating patients with chronic tinnitus--first results from a PET study. Acta Otolaryngol Suppl. 2006; (556):84–88.26. Schecklmann M, Landgrebe M, Poeppl TB, Kreuzer P, Männer P, Marienhagen J, et al. Neural correlates of tinnitus duration and distress: a positron emission tomography study. Hum Brain Mapp. 2013; 34:233–240.