Korean J Ophthalmol.
2015 Feb;29(1):47-52. 10.3341/kjo.2015.29.1.47.
Long-term Results from Cyclocryotherapy Applied to the 3O'clock and 9O'clock Positions in Blind Refractory Glaucoma Patients
- Affiliations
-
- 1Department of Ophthalmology, Gyeongsang National University School of Medicine, Jinju, Korea. maya12kim@naver.com
- 2Gyeongsang Institute of Health Science, Gyeongsang National University, Jinju, Korea.
- KMID: 2360137
- DOI: http://doi.org/10.3341/kjo.2015.29.1.47
Abstract
- PURPOSE
To report the long-term follow-up results after cyclocryotherapy, applied to the 3-o'clock and 9-o'clock positions in blind refractory glaucoma patients.
METHODS
We retrospectively reviewed the charts of 19 blind patients, and a total of 20 eyes with refractory glaucoma who were treated with cyclocryotherapy. Cyclocryotherapy treatments were performed using a retinal cryoprobe. The temperature of each cyclocryotherapy spot was -80degrees C and each spot was maintained in place for 60 seconds. Six cyclocryotherapy spots were placed in each quadrant, including the 3-o'clock and 9-o'clock positions.
RESULTS
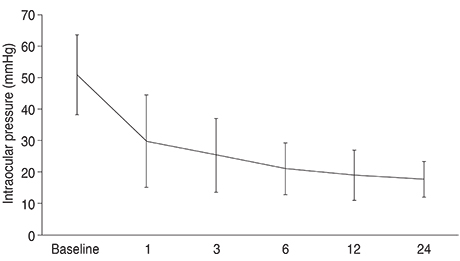
The mean baseline pretreatment intraocular pressure (IOP) in all eyes was 50.9 ± 12.5 mmHg, which significantly decreased to a mean IOP at last follow-up of 14.1 ± 7.1 mmHg (p < 0.001). The mean number of antiglaucoma medications that patients were still taking at last follow-up was 0.3 ± 0.6. Devastating post-procedure phthisis occurred in only one eye.
CONCLUSIONS
Cyclocryotherapy, performed at each quadrant and at the 3-o'clock and 9-o'clock position, is an effective way to lower IOP and, thus, is a reasonable treatment option for refractory glaucoma patients who experience with ocular pain and headaches.
Keyword
MeSH Terms
Figure
Reference
-
1. Bietti G. Surgical intervention on the ciliary body: new trends for the relief of glaucoma. J Am Med Assoc. 1950; 142:889–897.2. De Roetth A Jr. Cryosurgery for the treatment of advanced chronic simple glaucoma. Am J Ophthalmol. 1968; 66:1034–1041.3. Brancato R, Carassa RG, Bettin P, et al. Contact transscleral cyclophotocoagulation with diode laser in refractory glaucoma. Eur J Ophthalmol. 1995; 5:32–39.4. Hampton C, Shields MB, Miller KN, Blasini M. Evaluation of a protocol for transscleral neodymium: YAG cyclophotocoagulation in one hundred patients. Ophthalmology. 1990; 97:910–917.5. Krupin T, Mitchell KB, Becker B. Cyclocryotherapy in neovascular glaucoma. Am J Ophthalmol. 1978; 86:24–26.6. Streiff EB, Stucchi C. Antiglaucomatous cycloanemization: review of 207 operations performed during eight years. Am J Ophthalmol. 1966; 61(5 Pt 2):1325–1329.7. Sautter H, Demeler U. Antiglaucomatous ciliary body excision. Am J Ophthalmol. 1984; 98:344–348.8. Vogt A. Cyclodiathermypuncture in cases of glaucoma. Br J Ophthalmol. 1940; 24:288–297.9. Walton DS, Grant WM. Penetrating cyclodiathermy for filtration. Arch Ophthalmol. 1970; 83:47–48.10. Zarbin MA, Michels RG, de Bustros S, et al. Endolaser treatment of the ciliary body for severe glaucoma. Ophthalmology. 1988; 95:1639–1648.11. Finger PT, Perry HD, Shakin JL, et al. Microwave cyclodestruction: evaluation on human eyes. Br J Ophthalmol. 1995; 79:678–682.12. Bellows AR, Grant WM. Cyclocryotherapy of chronic open-angle glaucoma in aphakic eyes. Am J Ophthalmol. 1978; 85(5 Pt 1):615–621.13. Benson MT, Nelson ME. Cyclocryotherapy: a review of cases over a 10-year period. Br J Ophthalmol. 1990; 74:103–105.14. Hennekes R, Belgrado G. Cyclocryotherapy as an alternative treatment for primary glaucoma. Bull Soc Belge Ophtalmol. 1992; 244:169–176.15. Goldenberg-Cohen N, Bahar I, Ostashinski M, et al. Cyclocryotherapy versus transscleral diode laser cyclophotocoagulation for uncontrolled intraocular pressure. Ophthalmic Surg Lasers Imaging. 2005; 36:272–279.16. Caprioli J, Strang SL, Spaeth GL, Poryzees EH. Cyclocryotherapy in the treatment of advanced glaucoma. Ophthalmology. 1985; 92:947–954.17. Heuring AH, Hutz WW, Hoffmann PC, Eckhardt HB. Cyclocryotherapy in neovascular glaucoma and non-neovascular glaucoma. Klin Monbl Augenheilkd. 1998; 213:213–219.18. Ferry AP. Histopathologic observations on human eyes following cyclocryotherapy for glaucoma. Trans Sect Ophthalmol Am Acad Ophthalmol Otolaryngol. 1977; 83:90–113.19. Bellows AR. Cyclocryotherapy for glaucoma. Int Ophthalmol Clin. 1981; 21:99–111.20. Prost M. Cyclocryotherapy for glaucoma: evaluation of techniques. Surv Ophthalmol. 1983; 28:93–100.21. Brindley G, Shields MB. Value and limitations of cyclocryotherapy. Graefes Arch Clin Exp Ophthalmol. 1986; 224:545–548.22. Gibson RA. Reduction of corneal sensitivity after retinal detachment surgery. Br J Ophthalmol. 1981; 65:614–617.23. Martin XY, Safran AB. Corneal hypoesthesia. Surv Ophthalmol. 1988; 33:28–40.24. Muller LJ, Vrensen GF, Pels L, et al. Architecture of human corneal nerves. Invest Ophthalmol Vis Sci. 1997; 38:985–994.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Repeatability of Intraocular Pressure Patterns in Glaucomatous Patients
- Comparison of RNFL Thickness Measured by Two Different Kind of OCT in NTG Patients
- The Diurnal Variation and the Hypotensive Effect of Combined Therapy of Levobunolol and Dipivefrine in the Healthy Koreans and the Patients with Open Angle Glaucoma
- Effect of ERM on RNFL, GCIPL and Macular Thickness According to the Severity of Glaucoma
- Modified Rectangular Loop Suture for Refractory Pupillary Optic Capture of Scleral Fixated Intraocular Lens