J Korean Med Sci.
2016 Feb;31(2):280-285. 10.3346/jkms.2016.31.2.280.
Impaired Na+/K+-ATPase Function in Patients with Interstitial Cystitis/Painful Bladder Syndrome
- Affiliations
-
- 1Division of Urology, Department of Surgery, Taichung Armed Forces General Hospital, Taichung, Taiwan, Republic of China. jane.dar@yahoo.com.tw
- 2Central Taiwan University of Science and Technology, Taichung, Taiwan, Republic of China.
- 3National Defense Medical Center, Taipei, Taiwan, Republic of China.
- 4Department of Life Sciences, National Chung Hsing University, Taichung, Taiwan, Republic of China.
- 5Department of Urology, Feng-Yuan Hospital, Ministry of Health and Welfare, Taichung, Taiwan, Republic of China.
- KMID: 2360054
- DOI: http://doi.org/10.3346/jkms.2016.31.2.280
Abstract
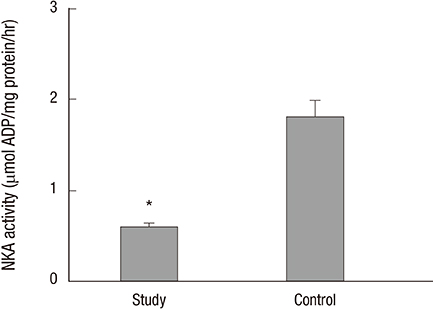
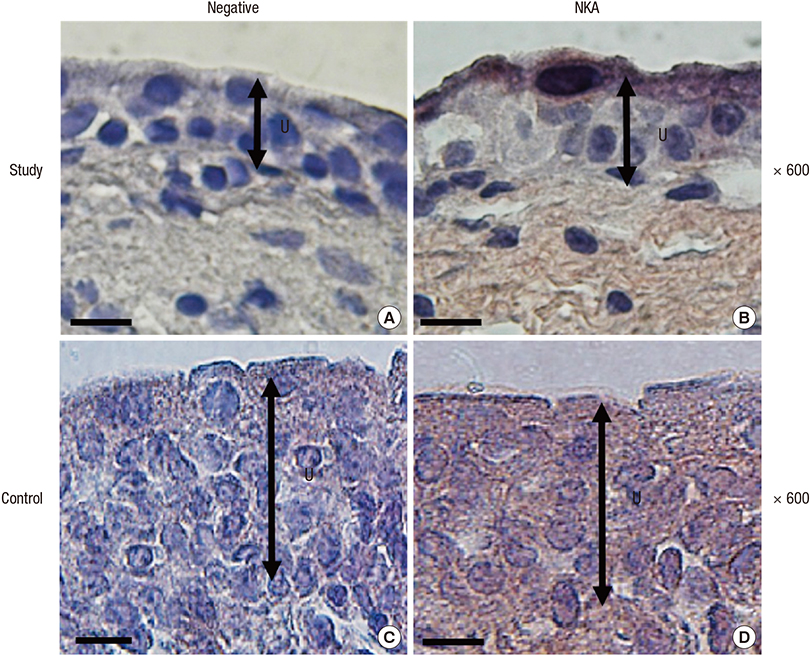
- Na+/K+-ATPase (NKA) is abundantly expressed in the basolateral membrane of epithelial cells, which is necessary for tight junction formation. The tight junction is an urothelial barrier between urine and the underlying bladder. Impairment of tight junctions allows migration of urinary solutes in patients with interstitial cystitis/painful bladder syndrome (IC/PBS). We evaluated NKA expression and activity in bladder samples from patients with IC/PBS. The study group consisted of 85 patients with IC/PBS, and the control group consisted of 20 volunteers. Bladder biopsies were taken from both groups. We determined the expression and distribution of NKA using NKA activity assays, immunoblotting, immunohistochemical staining, and immunofluorescent staining. The protein levels and activity of NKA in the study group were significantly lower than the control group (1.08 ± 0.06 vs. 2.39 ± 0.29 and 0.60 ± 0.04 vs. 1.81 ± 0.18 micromol ADP/mg protein/hour, respectively; P < 0.05). Additionally, immunofluorescent staining for detection of CK7, a marker of the bladder urothelium, predominantly colocalized with NKA in patients in the study group. Our results demonstrated the expression and activity of NKA were decreased in bladder biopsies of patients with IC/PBS. These findings suggest that NKA function is impaired in the bladders from patients with IC/PBS.
MeSH Terms
-
Adult
Cystitis, Interstitial/*diagnosis/metabolism
Female
Fluorescent Antibody Technique
Humans
Keratin-7/metabolism
Male
Microscopy, Fluorescence
Middle Aged
Sodium-Potassium-Exchanging ATPase/*metabolism
Urinary Bladder/metabolism/pathology
Urothelium/metabolism/pathology
Keratin-7
Sodium-Potassium-Exchanging ATPase
Figure
Reference
-
1. Brinson BE. Lactate dehydrogenase and Na+/K+ ATPase activity in Leiostomus xanthurus (spot) in response to hypoxia. Explorations. 2011; 4:22–29.2. Rajasekaran AK, Rajasekaran SA. Role of Na-K-ATPase in the assembly of tight junctions. Am J Physiol Renal Physiol. 2003; 285:F388–F396.3. Comellas AP, Dada LA, Lecuona E, Pesce LM, Chandel NS, Quesada N, Budinger GR, Strous GJ, Ciechanover A, Sznajder JI. Hypoxia-mediated degradation of Na,K-ATPase via mitochondrial reactive oxygen species and the ubiquitin-conjugating system. Circ Res. 2006; 98:1314–1322.4. Dada LA, Chandel NS, Ridge KM, Pedemonte C, Bertorello AM, Sznajder JI. Hypoxia-induced endocytosis of Na,K-ATPase in alveolar epithelial cells is mediated by mitochondrial reactive oxygen species and PKC-zeta. J Clin Invest. 2003; 111:1057–1064.5. Rajasekaran SA, Palmer LG, Moon SY, Peralta Soler A, Apodaca GL, Harper JF, Zheng Y, Rajasekaran AK. Na,K-ATPase activity is required for formation of tight junctions, desmosomes, and induction of polarity in epithelial cells. Mol Biol Cell. 2001; 12:3717–3732.6. Violette MI, Madan P, Watson AJ. Na+/K+ -ATPase regulates tight junction formation and function during mouse preimplantation development. Dev Biol. 2006; 289:406–419.7. Rajasekaran SA, Rajasekaran AK. Na,K-ATPase and epithelial tight junctions. Front Biosci (Landmark Ed). 2009; 14:2130–2148.8. Parsons CL. The role of the urinary epithelium in the pathogenesis of interstitial cystitis/prostatitis/urethritis. Urology. 2007; 69:Suppl. 9–16.9. Dell JR, Mokrzycki ML, Jayne CJ. Differentiating interstitial cystitis from similar conditions commonly seen in gynecologic practice. Eur J Obstet Gynecol Reprod Biol. 2009; 144:105–109.10. Fleming TP, Ghassemifar MR, Sheth B. Junctional complexes in the early mammalian embryo. Semin Reprod Med. 2000; 18:185–194.11. Zhang CO, Wang JY, Koch KR, Keay S. Regulation of tight junction proteins and bladder epithelial paracellular permeability by an antiproliferative factor from patients with interstitial cystitis. J Urol. 2005; 174:2382–2387.12. Hurst RE, Moldwin RM, Mulholland SG. Bladder defense molecules, urothelial differentiation, urinary biomarkers, and interstitial cystitis. Urology. 2007; 69:17–23.13. Lavelle JP, Meyers SA, Ruiz WG, Buffington CA, Zeidel ML, Apodaca G. Urothelial pathophysiological changes in feline interstitial cystitis: a human model. Am J Physiol Renal Physiol. 2000; 278:F540–F553.14. Parsons CL. The role of a leaky epithelium and potassium in the generation of bladder symptoms in interstitial cystitis/overactive bladder, urethral syndrome, prostatitis and gynaecological chronic pelvic pain. BJU Int. 2011; 107:370–375.15. Gillenwater JY, Wein AJ. Summary of the National Institute of Arthritis, Diabetes, Digestive and Kidney Diseases Workshop on Interstitial Cystitis, National Institutes of Health, Bethesda, Maryland, August 28-29, 1987. J Urol. 1988; 140:203–206.16. Evans RJ, Sant GR. Current diagnosis of interstitial cystitis: an evolving paradigm. Urology. 2007; 69:64–72.17. Ottem DP, Teichman JM. What is the value of cystoscopy with hydrodistension for interstitial cystitis? Urology. 2005; 66:494–499.18. Lee JD, Lee MH. Activation of extrinsic apoptotic pathway from bladder biopsy in patients with interstitial cystitis/painful bladder syndrome. Urology. 2013; 82:1451.e7–1451.11.19. Lee JD, Lee MH. Decreased expression of zonula occludens-1 and occludin in the bladder urothelium of patients with interstitial cystitis/painful bladder syndrome. J Formos Med Assoc. 2014; 113:17–22.20. Lee JD, Lee MH. Increased expression of hypoxia-inducible factor-1α and vascular endothelial growth factor associated with glomerulation formation in patients with interstitial cystitis. Urology. 2011; 78:971.e11–971.e15.21. Yang WK, Kang CK, Chen TY, Chang WB, Lee TH. Salinity-dependent expression of the branchial Na+/K +/2Cl- cotransporter and Na+/K+-ATPase in the sailfin molly correlates with hypoosmoregulatory endurance. J Comp Physiol B. 2011; 181:953–964.22. Haghighi K, Pritchard T, Bossuyt J, Waggoner JR, Yuan Q, Fan GC, Osinska H, Anjak A, Rubinstein J, Robbins J, et al. The human phospholamban Arg14-deletion mutant localizes to plasma membrane and interacts with the Na/K-ATPase. J Mol Cell Cardiol. 2012; 52:773–782.23. Lee JD, Lee MH. Metallothionein overexpression of bladder biopsies associated with tissue hypoxia in patients with interstitial cystitis/painful bladder syndrome. Int J Urol. 2014; 21:719–723.24. Smith PR, Mackler SA, Weiser PC, Brooker DR, Ahn YJ, Harte BJ, McNulty KA, Kleyman TR. Expression and localization of epithelial sodium channel in mammalian urinary bladder. Am J Physiol. 1998; 274:F91–F96.25. Espineda C, Seligson DB, James Ball W Jr, Rao J, Palotie A, Horvath S, Huang Y, Shi T, Rajasekaran AK. Analysis of the Na,K-ATPase alpha- and beta-subunit expression profiles of bladder cancer using tissue microarrays. Cancer. 2003; 97:1859–1868.26. Spector DA, Deng J, Coleman R, Wade JB. The urothelium of a hibernator: the American black bear. Physiol Rep. 2015; 3:e12429.27. Shie JH, Kuo HC. Higher levels of cell apoptosis and abnormal E-cadherin expression in the urothelium are associated with inflammation in patients with interstitial cystitis/painful bladder syndrome. BJU Int. 2011; 108:E136–E141.28. Rajasekaran SA, Barwe SP, Gopal J, Ryazantsev S, Schneeberger EE, Rajasekaran AK. Na-K-ATPase regulates tight junction permeability through occludin phosphorylation in pancreatic epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2007; 292:G124–G133.29. Rajasekaran SA, Hu J, Gopal J, Gallemore R, Ryazantsev S, Bok D, Rajasekaran AK. Na,K-ATPase inhibition alters tight junction structure and permeability in human retinal pigment epithelial cells. Am J Physiol Cell Physiol. 2003; 284:C1497–C1507.30. Hauser PJ, Dozmorov MG, Bane BL, Slobodov G, Culkin DJ, Hurst RE. Abnormal expression of differentiation related proteins and proteoglycan core proteins in the urothelium of patients with interstitial cystitis. J Urol. 2008; 179:764–769.31. Erickson DR, Schwarze SR, Dixon JK, Clark CJ, Hersh MA. Differentiation associated changes in gene expression profiles of interstitial cystitis and control urothelial cells. J Urol. 2008; 180:2681–2687.32. Keay S. Cell signaling in interstitial cystitis/painful bladder syndrome. Cell Signal. 2008; 20:2174–2179.33. Panayiotidis MI, Bortner CD, Cidlowski JA. On the mechanism of ionic regulation of apoptosis: would the Na+/K+-ATPase please stand up? Acta Physiol (Oxf). 2006; 187:205–215.34. Zhou G, Dada LA, Sznajder JI. Regulation of alveolar epithelial function by hypoxia. Eur Respir J. 2008; 31:1107–1113.35. Gusarova GA, Trejo HE, Dada LA, Briva A, Welch LC, Hamanaka RB, Mutlu GM, Chandel NS, Prakriya M, Sznajder JI. Hypoxia leads to Na,K-ATPase downregulation via Ca2+ release-activated Ca2+ channels and AMPK activation. Mol Cell Biol. 2011; 31:3546–3556.36. Wheaton WW, Chandel NS. Hypoxia. 2. Hypoxia regulates cellular metabolism. Am J Physiol Cell Physiol. 2011; 300:C385–93.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Interstitial Cystitis/Painful Bladder Syndrome: Prevalence Estimates, Quality of Life and Depression among Older Adult Korean Women
- Diagnosis and treatment of interstitial cystitis and painful bladder syndrome
- Antiproliferative Factor Signaling and Interstitial Cystitis/Painful Bladder Syndrome
- Diagnosis and Management of Interstitial Cystitis/ Painful Bladder Syndrome
- Potential Use of Antiproliferative Factor as a Targeting Therapy in Painful Bladder Syndrome/Interstitial Cystitis