J Korean Med Sci.
2016 Feb;31(2):214-221. 10.3346/jkms.2016.31.2.214.
Predictive Factors of Mortality in Population of Patients with Paroxysmal Nocturnal Hemoglobinuria (PNH): Results from a Korean PNH Registry
- Affiliations
-
- 1Division of Hematology-Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 2Divison of Hematology, Department of Internal Medicine, Yonsei University College of Medicine, Severance Hospital, Seoul, Korea.
- 3Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea.
- 4Department of Hematology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 5Department of Hematology-Oncology, Chonnam National University Hwasun Hospital, Hwasun, Korea.
- 6Department of Internal Medicine, Chungnam National University Hospital, Chungnam National University College of Medicine, Daejeon, Korea.
- 7Division of Hematology-Oncology, Department of Internal Medicine, Pusan National University Hospital, Pusan National University School of Medicine, Busan, Korea.
- 8Department of Hematology/Oncology, Kyungpook National University School of Medicine, Daegu, Korea.
- 9Division of Hematology, Department of Internal Medicine, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea. jwlee@catholic.ac.kr
- KMID: 2360045
- DOI: http://doi.org/10.3346/jkms.2016.31.2.214
Abstract
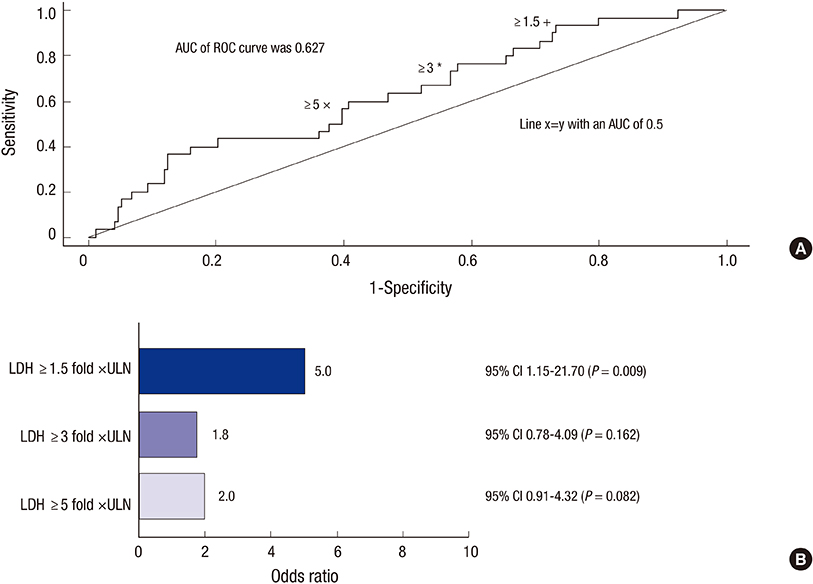
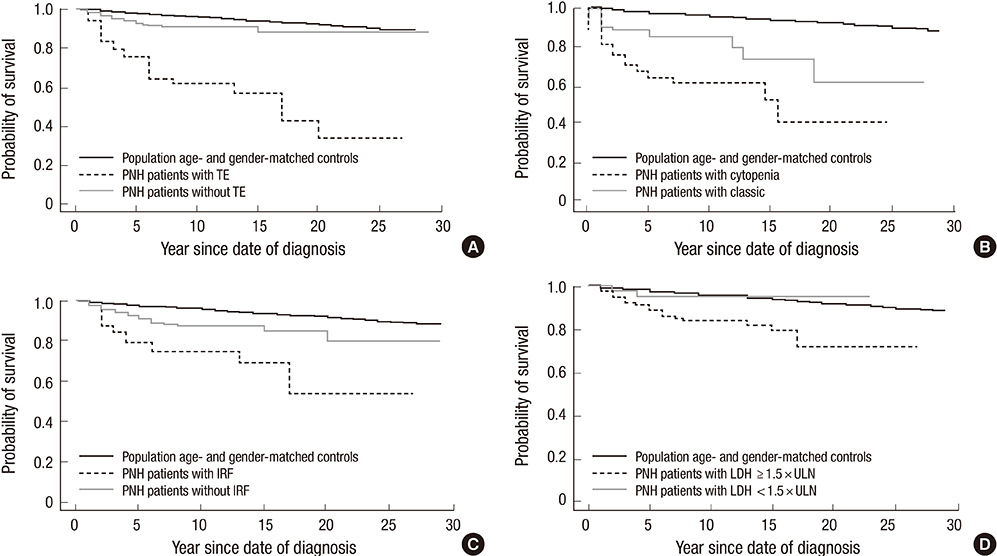
- Paroxysmal nocturnal hemoglobinuria (PNH) is a progressive, systemic, life-threatening disease, characterized by chronic uncontrolled complement activation. A retrospective analysis of 301 Korean PNH patients who had not received eculizumab was performed to systematically identify the clinical symptoms and signs predictive of mortality. PNH patients with hemolysis (lactate dehydrogenase [LDH] > or = 1.5 x the upper limit of normal [ULN]) have a 4.8-fold higher mortality rate compared with the age- and sex-matched general population (P < 0.001). In contrast, patients with LDH < 1.5 x ULN have a similar mortality rate as the general population (P = 0.824). Thromboembolism (TE) (odds ratio [OR] 7.11; 95% confidence interval [CI] (3.052-16.562), renal impairment (OR, 2.953; 95% CI, 1.116-7.818) and PNH-cytopenia (OR, 2.547; 95% CI, 1.159-5.597) are independent risk factors for mortality, with mortality rates 14-fold (P < 0.001), 8-fold (P < 0.001), and 6.2-fold (P < 0.001) greater than that of the age- and sex-matched general population, respectively. The combination of hemolysis and 1 or more of the clinical symptoms such as abdominal pain, chest pain, or dyspnea, resulted in a much greater increased mortality rate when compared with patients with just the individual symptom alone or just hemolysis. Early identification of risk factors related to mortality is crucial for the management of PNH. This trial was registered at www.clinicaltrials.gov as NCT01224483.
MeSH Terms
-
Adolescent
Adult
Aged
Aged, 80 and over
Antibodies, Monoclonal/therapeutic use
Antibodies, Monoclonal, Humanized/therapeutic use
Area Under Curve
Child
Dyspnea/etiology
Female
Hemoglobinuria, Paroxysmal/*diagnosis/drug therapy/mortality
Hemolysis
Humans
Kaplan-Meier Estimate
Kidney Diseases/complications/diagnosis
L-Lactate Dehydrogenase/metabolism
Male
Middle Aged
Odds Ratio
ROC Curve
Registries
Republic of Korea
Retrospective Studies
Risk Factors
Thromboembolism/complications/diagnosis
Young Adult
Antibodies, Monoclonal
Antibodies, Monoclonal, Humanized
L-Lactate Dehydrogenase
Figure
Cited by 1 articles
-
Efficacy of eculizumab in paroxysmal nocturnal hemoglobinuria patients with or without aplastic anemia: prospective study of a Korean PNH cohort
Chul Won Choi, Jun Ho Jang, Jin Seok Kim, Deog-Yeon Jo, Je-Hwan Lee, Sung-Hyun Kim, Yeo-Kyeoung Kim, Jong-Ho Won, Joo Seop Chung, Hawk Kim, Jae Hoon Lee, Min Kyoung Kim, Hyeon-Seok Eom, Shin Young Hyun, Jeong-A Kim, Jong Wook Lee
Blood Res. 2017;52(3):207-211. doi: 10.5045/br.2017.52.3.207.
Reference
-
1. Hillmen P, Lewis SM, Bessler M, Luzzatto L, Dacie JV. Natural history of paroxysmal nocturnal hemoglobinuria. N Engl J Med. 1995; 333:1253–1259.2. Socié G, Mary JY, de Gramont A, Rio B, Leporrier M, Rose C, Heudier P, Rochant H, Cahn JY, Gluckman E. French Society of Haematology. Paroxysmal nocturnal haemoglobinuria: long-term follow-up and prognostic factors. Lancet. 1996; 348:573–577.3. Hill A, Kelly RJ, Hillmen P. Thrombosis in paroxysmal nocturnal hemoglobinuria. Blood. 2013; 121:4985–4996.4. Lee JW, Jang JH, Kim JS, Yoon SS, Lee JH, Kim YK, Jo DY, Chung J, Sohn SK. Clinical signs and symptoms associated with increased risk for thrombosis in patients with paroxysmal nocturnal hemoglobinuria from a Korean Registry. Int J Hematol. 2013; 97:749–757.5. Hillmen P, Elebute M, Kelly R, Urbano-Ispizua A, Hill A, Rother RP, Khursigara G, Fu CL, Omine M, Browne P, et al. Long-term effect of the complement inhibitor eculizumab on kidney function in patients with paroxysmal nocturnal hemoglobinuria. Am J Hematol. 2010; 85:553–559.6. Hillmen P, Muus P, Dührsen U, Risitano AM, Schubert J, Luzzatto L, Schrezenmeier H, Szer J, Brodsky RA, Hill A, et al. Effect of the complement inhibitor eculizumab on thromboembolism in patients with paroxysmal nocturnal hemoglobinuria. Blood. 2007; 110:4123–4128.7. Kelly RJ, Hill A, Arnold LM, Brooksbank GL, Richards SJ, Cullen M, Mitchell LD, Cohen DR, Gregory WM, Hillmen P. Long-term treatment with eculizumab in paroxysmal nocturnal hemoglobinuria: sustained efficacy and improved survival. Blood. 2011; 117:6786–6792.8. Parker C, Omine M, Richards S, Nishimura J, Bessler M, Ware R, Hillmen P, Luzzatto L, Young N, Kinoshita T, et al. Diagnosis and management of paroxysmal nocturnal hemoglobinuria. Blood. 2005; 106:3699–3709.9. Hillmen P, Hall C, Marsh JC, Elebute M, Bombara MP, Petro BE, Cullen MJ, Richards SJ, Rollins SA, Mojcik CF, et al. Effect of eculizumab on hemolysis and transfusion requirements in patients with paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2004; 350:552–559.10. Hillmen P, Young NS, Schubert J, Brodsky RA, Socié G, Muus P, Röth A, Szer J, Elebute MO, Nakamura R, et al. The complement inhibitor eculizumab in paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2006; 355:1233–1243.11. Brodsky RA, Young NS, Antonioli E, Risitano AM, Schrezenmeier H, Schubert J, Gaya A, Coyle L, de Castro C, Fu CL, et al. Multicenter phase 3 study of the complement inhibitor eculizumab for the treatment of patients with paroxysmal nocturnal hemoglobinuria. Blood. 2008; 111:1840–1847.12. de Latour RP, Mary JY, Salanoubat C, Terriou L, Etienne G, Mohty M, Roth S, de Guibert S, Maury S, Cahn JY, et al. Paroxysmal nocturnal hemoglobinuria: natural history of disease subcategories. Blood. 2008; 112:3099–3106.13. Nishimura J, Kanakura Y, Ware RE, Shichishima T, Nakakuma H, Ninomiya H, Decastro CM, Hall S, Kanamaru A, Sullivan KM, et al. Clinical course and flow cytometric analysis of paroxysmal nocturnal hemoglobinuria in the United States and Japan. Medicine (Baltimore). 2004; 83:193–207.14. Hill A, Sapsford RJ, Scally A, Kelly R, Richards SJ, Khurisgara G, Sivananthan MU, Hillmen P. Under-recognized complications in patients with paroxysmal nocturnal haemoglobinuria: raised pulmonary pressure and reduced right ventricular function. Br J Haematol. 2012; 158:409–414.15. Schrezenmeier H, Muus P, Socié G, Szer J, Urbano-Ispizua A, Maciejewski JP, Brodsky RA, Bessler M, Kanakura Y, Rosse W, et al. Baseline characteristics and disease burden in patients in the International Paroxysmal Nocturnal Hemoglobinuria Registry. Haematologica. 2014; 99:922–929.16. Brodsky RA. Advances in the diagnosis and therapy of paroxysmal nocturnal hemoglobinuria. Blood Rev. 2008; 22:65–74.17. Mukhina GL, Buckley JT, Barber JP, Jones RJ, Brodsky RA. Multilineage glycosylphosphatidylinositol anchor-deficient haematopoiesis in untreated aplastic anaemia. Br J Haematol. 2001; 115:476–482.18. Ge M, Li X, Shi J, Shao Y, Zheng Y. Clinical features and prognostic factors of Asian patients with paroxysmal nocturnal hemoglobinuria: results from a single center in China. Ann Hematol. 2012; 91:1121–1128.19. Hill A, Hillmen P, Richards SJ, Elebute D, Marsh JC, Chan J, Mojcik CF, Rother RP. Sustained response and long-term safety of eculizumab in paroxysmal nocturnal hemoglobinuria. Blood. 2005; 106:2559–2565.20. Weitz I, Meyers G, Lamy T, Cahn JY, Uranga MT, García Vela JA, Sanz MA, Severino B, Kelly RJ, Hillmen P, et al. Cross-sectional validation study of patient-reported outcomes in patients with paroxysmal nocturnal haemoglobinuria. Intern Med J. 2013; 43:298–307.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Paroxysmal Nocturnal Hemoglobinuria Presenting as Recurrent Jejunitis
- Two Cases of Acute Renal Failure Complicating Paroxysmal Nocturnal Hemoglobinuria in Children
- Comparison of High Sensitivity and Conventional Flow Cytometry for Diagnosing Overt Paroxysmal Nocturnal Hemoglobinuria and Detecting Minor Paroxysmal Nocturnal Hemoglobinuria Clones
- Progressive Moyamoya Syndrome Associated with Paroxysmal Nocturnal Hemoglobinuria
- Anesthetic and Perioperative Management of Patient with Paroxysmal Nocturnal Hemoglobinuria