J Korean Med Sci.
2015 Dec;30(12):1815-1820. 10.3346/jkms.2015.30.12.1815.
Clinical Features of Drug-induced Liver Injury According to Etiology
- Affiliations
-
- 1Institute for Digestive Research, Digestive Disease Center, Department of Internal Medicine, College of Medicine, Soonchunhyang University, Seoul, Korea. jyjang@schmc.ac.kr
- 2Department of Internal Medicine, College of Medicine, Soonchunhyang University, Cheonan, Korea.
- 3Department of Internal Medicine, College of Medicine, Soonchunhyang University, Bucheon, Korea.
- KMID: 2359966
- DOI: http://doi.org/10.3346/jkms.2015.30.12.1815
Abstract
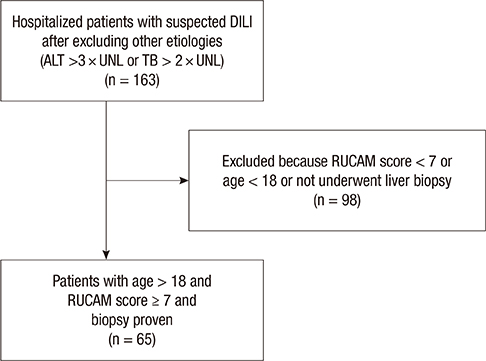
- Drug-induced liver injury (DILI) is an increasingly common cause of acute hepatitis. We examined clinical features and types of liver injury of 65 affected patients who underwent liver biopsy according DILI etiology. The major causes of DILI were the use of herbal medications (43.2%), prescribed medications (21.6%), and traditional therapeutic preparations and dietary supplements (35%). DILI from herbal medications, traditional therapeutic preparations, and dietary supplements was associated with higher elevations in aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels than was DILI from prescription medications. The types of liver injury based on the R ratio were hepatocellular (67.7%), mixed (10.8%), and cholestatic (21.5%). Herbal medications and traditional therapeutic preparations were more commonly associated with hepatocellular liver injury than were prescription medications (P = 0.002). Herbal medications and traditional therapeutic preparations induce more hepatocellular DILI and increased elevations in AST and ALT than prescribed medications.
Keyword
MeSH Terms
-
Adult
Alanine Transaminase/blood
Aspartate Aminotransferases/blood
Dietary Supplements/adverse effects
Drug-Induced Liver Injury/enzymology/*etiology/pathology
Female
Humans
Male
Middle Aged
Phytotherapy/adverse effects
Plant Preparations/adverse effects
Prescription Drugs/adverse effects
Republic of Korea
Retrospective Studies
Alanine Transaminase
Aspartate Aminotransferases
Plant Preparations
Prescription Drugs
Figure
Reference
-
1. Vuppalanchi R, Liangpunsakul S, Chalasani N. Etiology of new-onset jaundice: how often is it caused by idiosyncratic drug-induced liver injury in the United States? Am J Gastroenterol. 2007; 102:558–562.2. Ostapowicz G, Fontana RJ, Schiødt FV, Larson A, Davern TJ, Han SH, McCashland TM, Shakil AO, Hay JE, Hynan L, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002; 137:947–954.3. Suk KT, Kim DJ. Drug-induced liver injury: present and future. Clin Mol Hepatol. 2012; 18:249–257.4. Au JS, Navarro VJ, Rossi S. Review article: Drug-induced liver injury--its pathophysiology and evolving diagnostic tools. Aliment Pharmacol Ther. 2011; 34:11–20.5. Andrade RJ, Lucena MI, Fernández MC, Pelaez G, Pachkoria K, Garcia-Ruiz E, García-Muñoz B, González-Grande R, Pizarro A, Duán JA, et al. Drug-induced liver injury: an analysis of 461 incidences submitted to the Spanish registry over a 10-year period. Gastroenterology. 2005; 129:512–521.6. Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J. Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008; 135:1924–1934. 1934.e1–1934.e4.7. Ju HY, Jang JY, Jeong SW, Woo SA, Kong MG, Jang HY, Lee SH, Kim SG, Cha SW, Kim YS, et al. The clinical features of drug-induced liver injury observed through liver biopsy: focus on relevancy to autoimmune hepatitis. Clin Mol Hepatol. 2012; 18:213–218.8. Reuben A. Hy's law. Hepatology. 2004; 39:574–578.9. Fontana RJ, Seeff LB, Andrade RJ, Björnsson E, Day CP, Serrano J, Hoofnagle JH. Standardization of nomenclature and causality assessment in drug-induced liver injury: summary of a clinical research workshop. Hepatology. 2010; 52:730–742.10. Suk KT, Kim DJ, Kim CH, Park SH, Yoon JH, Kim YS, Baik GH, Kim JB, Kweon YO, Kim BI, et al. A prospective nationwide study of drug-induced liver injury in Korea. Am J Gastroenterol. 2012; 107:1380–1387.11. De Valle MB, Av Klinteberg V, Alem N, Olsson R, Björnsson E. Drug-induced liver injury in a Swedish University hospital out-patient hepatology clinic. Aliment Pharmacol Ther. 2006; 24:1187–1195.12. Teschke R, Schulze J, Schwarzenboeck A, Eickhoff A, Frenzel C. Herbal hepatotoxicity: suspected cases assessed for alternative causes. Eur J Gastroenterol Hepatol. 2013; 25:1093–1098.13. Bunchorntavakul C, Reddy KR. Review article: herbal and dietary supplement hepatotoxicity. Aliment Pharmacol Ther. 2013; 37:3–17.14. Xu HM, Chen Y, Xu J, Zhou Q. Drug-induced liver injury in hospitalized patients with notably elevated alanine aminotransferase. World J Gastroenterol. 2012; 18:5972–5978.15. Devarbhavi H, Dierkhising R, Kremers WK, Sandeep MS, Karanth D, Adarsh CK. Single-center experience with drug-induced liver injury from India: causes, outcome, prognosis, and predictors of mortality. Am J Gastroenterol. 2010; 105:2396–2404.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Drug Induced Liver Injury by Prophylactic Administration of Albendazole
- Factors affecting drug-induced liver injury: antithyroid drugs as instances
- Drug-induced liver injury
- Minocycline-Induced Autoimmune Hepatitis: A Rare But Important Cause of Drug-Induced Autoimmune Hepatitis
- Ombitasvir/paritaprevir/ritonavir+dasabuvir and ribavirin associated drug-induced liver injury and syndrome of inappropriate secretion of anti-diuretic hormone: A case report