Nutr Res Pract.
2016 Dec;10(6):583-589. 10.4162/nrp.2016.10.6.583.
Effect of dual-type oligosaccharides on constipation in loperamide-treated rats
- Affiliations
-
- 1Bk21Plus, College of Health Science, Korea University, Seoul 02841, Korea.
- 2Institute for Biomaterials, Korea University, Seoul 02841, Korea.
- 3Department of Public Health Sciences, Graduate School, Korea University, 145 Anam-ro, Sungbuk-gu, Seoul 02841, Korea. suh1960@korea.ac.kr
- KMID: 2359614
- DOI: http://doi.org/10.4162/nrp.2016.10.6.583
Abstract
- BACKGROUND/OBJECTIVES
Constipation is a condition that can result from intestinal deformation. Because humans have an upright posture, the effects of gravity can cause this shape deformation. Oligosaccharides are common prebiotics and their effects on bowel health are well known. However, studies of the physiological functionality of a product that contains both lactulose and galactooligosaccharides are insufficient. We investigated the constipation reduction effect of a dual-type oligosaccharide, Dual-Oligo, in loperamide-treated rats.
MATERIALS/METHODS
Dual-Oligo consists of galactooligosaccharides (15.80%) and lactulose (51.67%). Animals were randomly divided into four groups, the normal group (normal), control group (control), low concentration of Dual-Oligo (LDO) group, and high concentration of Dual-Oligo (HDO) group. After 7 days of oral administration, fecal pellet amount, fecal weight, water content of fecal were measured. Blood chemistry, short-chain fatty acid (SCFA), gastrointestinal transit ratio and length and intestinal mucosa were analyzed.
RESULTS
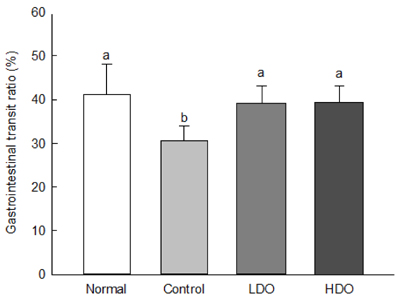
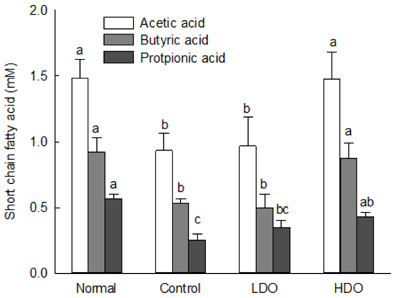
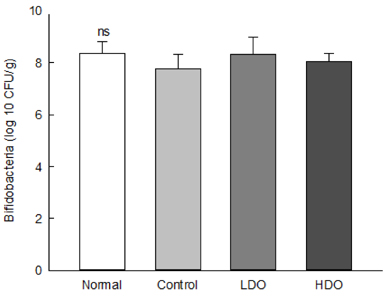
Dual-Oligo increased the fecal weight, and water content of feces in rats with loperamide-induced constipation. Gastrointestinal transit ratio and length and area of intestinal mucosa significantly increased after treatment with Dual-Oligo in loperamide-induced rats. A high concentration of Dual-Oligo tended to produce more acetic acid than that observed for the control group, and Dual-Oligo affected the production of total SCFA. Bifidobacteria concentration of cecal contents in the high-concentration oligosaccharide (HDO) and low-concentration oligosaccharide (LDO) groups was similar to the result of the normal group.
CONCLUSIONS
These results showed that Dual-Oligo is a functional material that is derived from a natural food product and is effective in ameliorating constipation.
MeSH Terms
Figure
Reference
-
1. Harari D, Gurwitz JH, Minaker KL. Constipation in the elderly. J Am Geriatr Soc. 1993; 41:1130–1140.
Article2. Müller-Lissner S, Rykx A, Kerstens R, Vandeplassche L. A double-blind, placebo-controlled study of prucalopride in elderly patients with chronic constipation. Neurogastroenterol Motil. 2010; 22:991–998. e255.
Article3. Gallagher PF, O'Mahony D, Quigley EM. Management of chronic constipation in the elderly. Drugs Aging. 2008; 25:807–821.
Article4. Kallman H. Constipation in the elderly. Am Fam Physician. 1983; 27:179–184.5. Faigel DO. A clinical approach to constipation. Clin Cornerstone. 2002; 4:11–21.
Article6. Lacy BE, Brunton SA. Partnering with gastroenterologists to evaluate patients with chronic constipation. MedGenMed. 2005; 7:19.7. Smith B. Effect of irritant purgatives on the myenteric plexus in man and the mouse. Gut. 1968; 9:139–143.
Article8. Smith B. Pathologic changes in the colon produced by anthraquinone purgatives. Dis Colon Rectum. 1973; 16:455–458.
Article9. Ly SY, Cho JH, Lee KA. Effects of fructooligosaccharide and fructooligosaccharide containing sponge cake on blood lipids, intestinal function and short chain fatty acid production in rats. Korean J Nutr. 2003; 36:344–351.10. Yu LL, Liao JF, Chen CF. Anti-diarrheal effect of water extract of Evodiae fructus in mice. J Ethnopharmacol. 2000; 73:39–45.
Article11. Demigné C, Rémésy C. Stimulation of absorption of volatile fatty acids and minerals in the cecum of rats adapted to a very high fiber diet. J Nutr. 1985; 115:53–60.
Article12. Guillon F, Champ MM. Carbohydrate fractions of legumes: uses in human nutrition and potential for health. Br J Nutr. 2002; 88:Suppl 3. S293–S306.
Article13. Corfield AP, Carroll D, Myerscough N, Probert CS. Mucins in the gastrointestinal tract in health and disease. Front Biosci. 2001; 6:D1321–D1357.
Article14. Hsieh C. Treatment of constipation in older adults. Am Fam Physician. 2005; 72:2277–2284.15. Potter J, Wagg A. Management of bowel problems in older people: an update. Clin Med (Lond). 2005; 5:289–295.
Article16. Kim HJ, Kim SI, Han YS. Effects of sea tangle extract and sea tangle yogurt on constipation relief. Korean J Food Cookery Sci. 2008; 24:59–67.17. Wintola OA, Sunmonu TO, Afolayan AJ. The effect of Aloe ferox Mill. in the treatment of loperamide-induced constipation in Wistar rats. BMC Gastroenterol. 2010; 10:95.
Article18. Lee HY, Kim JH, Jeung HW, Lee CU, Kim DS, Li B, Lee GH, Sung MS, Ha KC, Back HI, Kim SY, Park SH, Oh MR, Kim MG, Jeon JY, Im YJ, Hwang MH, So BO, Shin SJ, Yoo WH, Kim HR, Chae HJ, Chae SW. Effects of Ficus carica paste on loperamide-induced constipation in rats. Food Chem Toxicol. 2012; 50:895–902.
Article19. Shimotoyodome A, Meguro S, Hase T, Tokimitsu I, Sakata T. Decreased colonic mucus in rats with loperamide-induced constipation. Comp Biochem Physiol A Mol Integr Physiol. 2000; 126:203–212.
Article20. Huang H, Jiang Q, Chen Y, Li X, Mao X, Chen X, Huang L, Gao W. Preparation, physico-chemical characterization and biological activities of two modified starches from yam (Dioscorea Opposita Thunb.). Food Hydrocoll. 2016; 55:244–253.
Article21. Aït-Aissa A, Aïder M. Lactulose: production and use in functional food, medical and pharmaceutical applications. Practical and critical review. Int J Food Sci Technol. 2014; 49:1245–1253.
Article22. Sakata T. Stimulatory effect of short-chain fatty acids on epithelial cell proliferation of isolated and denervated jejunal segment of the rat. Scand J Gastroenterol. 1989; 24:886–890.
Article23. Frankenhaeuser M. Behavior and circulating catecholamines. Brain Res. 1971; 31:241–262.
Article24. Sin HJ, Kim KO, Kim SH, Kim YA, Lee HS. Effect of resistant starch on the large bowel environment and plasma lipid in rats with loperamide-induced constipation. J Korean Soc Food Sci Nutr. 2010; 39:684–691.
Article25. Thompson DB. Strategies for the manufacture of resistant starch. Trends Food Sci Technol. 2000; 11:245–253.
Article26. Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995; 125:1401–1412.
Article27. Cummings JH, Macfarlane GT, Drasar BS. The gut microflora and its significance. In : Whitehead R, editor. Gastrointestinal and Oesophageal Pathology. Edinburgh: Churchill Livingstone;1989. p. 201–219.28. Tancrède C. Role of human microflora in health and disease. Eur J Clin Microbiol Infect Dis. 1992; 11:1012–1015.
Article29. Mutai M, Tanaka R. Ecology of Bifidobacterium in the human intestinal flora. Bifidobact Microflora. 1987; 6:33–41.
Article30. Bezkorovainy A, Miller-Catchpole R. Biochemistry and Physiology of Bifidobacteria. Boca Raton (FL): CRC press;1989.31. Hong KB, Suh HJ, Kim JH, Kwon HK, Park C, Han SH. Preparation of high purity galacto-oligosaccharide and its prebiotic activity in vitro evaluation. Korean J Food Nutr. 2015; 28:1026–1032.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Probiotic on Constipation in Rats
- Correlation between laxative effects of uridine and suppression of ER stress in loperamide induced constipated SD rats
- Metabolomics approach to serum biomarker for loperamide-induced constipation in SD rats
- Laxative effect of peanut sprout extract
- Laxative effects of Liriope platyphylla are tightly correlated with suppression of endoplasmic reticulum stress in loperamide-induced constipation of SD rats