Brain Tumor Res Treat.
2016 Oct;4(2):77-86. 10.14791/btrt.2016.4.2.77.
Impact of Human Immunodeficiency Virus in the Pathogenesis and Outcome of Patients with Glioblastoma Multiforme
- Affiliations
-
- 1Department of Neurosurgery, University of California, Los Angeles, Los Angeles, CA, USA. iyang@mednet.ucla.edu
- 2Jonsson Comprehensive Cancer Center, University of California, Los Angeles, Los Angeles, CA, USA.
- KMID: 2356974
- DOI: http://doi.org/10.14791/btrt.2016.4.2.77
Abstract
- BACKGROUND
Improvement in antiviral therapies have been accompanied by an increased frequency of non-Acquired Immune Deficiency Syndrome (AIDS) defining malignancies, such as glioblastoma multiforme. Here, we investigated all reported cases of human immunodeficiency virus (HIV)-positive patients with glioblastoma and evaluated their clinical outcomes. A comprehensive review of the molecular pathogenetic mechanisms underlying glioblastoma development in the setting of HIV/AIDS is provided.
METHODS
We performed a PubMed search using keywords "HIV glioma" AND "glioblastoma," and "AIDS glioma" AND "glioblastoma." Case reports and series describing HIV-positive patients with glioblastoma (histologically-proven World Health Organization grade IV astrocytoma) and reporting on HAART treatment status, clinical follow-up, and overall survival (OS), were included for the purposes of quantitative synthesis. Patients without clinical follow-up data or OS were excluded. Remaining articles were assessed for data extraction eligibility.
RESULTS
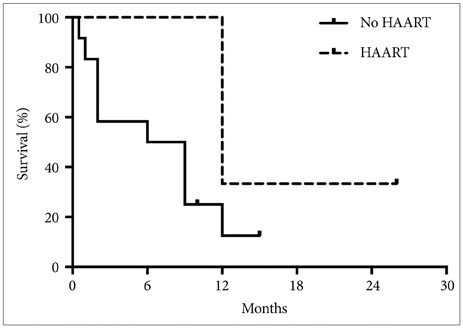
A total of 17 patients met our inclusion criteria. Of these patients, 14 (82.4%) were male and 3 (17.6%) were female, with a mean age of 39.5±9.2 years (range 19-60 years). Average CD4 count at diagnosis of glioblastoma was 358.9±193.4 cells/mm3. Tumor progression rather than AIDS-associated complications dictated patient survival. There was a trend towards increased median survival with HAART treatment (12.0 vs 7.5 months, p=0.10)
CONCLUSION
Our data suggests that HAART is associated with improved survival in patients with HIV-associated glioblastoma, although the precise mechanisms underlying this improvement remain unclear.
Keyword
MeSH Terms
Figure
Reference
-
1. UNAIDS [Internet]. Global report: UNAIDS report on the global AIDS epidemic 2010. Geneva: Available from: http://www.unaids.org/globalreport/Global_report.htm.2. Palella FJ Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998; 338:853–860.
Article3. Lohse N, Hansen AB, Gerstoft J, Obel N. Improved survival in HIV-infected persons: consequences and perspectives. J Antimicrob Chemother. 2007; 60:461–463.
Article4. Ohgaki H, Kleihues P. Population-based studies on incidence, survival rates, and genetic alterations in astrocytic and oligodendroglial gliomas. J Neuropathol Exp Neurol. 2005; 64:479–489.
Article5. Krex D, Klink B, Hartmann C, et al. Long-term survival with glioblastoma multiforme. Brain. 2007; 130(Pt 10):2596–2606.
Article6. Lawson HC, Sampath P, Bohan E, et al. Interstitial chemotherapy for malignant gliomas: the Johns Hopkins experience. J Neurooncol. 2007; 83:61–70.
Article7. Weill O, Finaud M, Bille F, et al. [Malignant spinal cord glioma. A new complication of HIV virus infection?]. Presse Med. 1987; 16:1977.8. Rosenblum ML, Levy RM, Bredesen DE. Neurosurgical implications of the acquired immunodeficiency syndrome (AIDS). Clin Neurosurg. 1988; 34:419–445.9. Monfardini S, Vaccher E, Pizzocaro G, et al. Unusual malignant tumours in 49 patients with HIV infection. AIDS. 1989; 3:449–452.
Article10. Wolff R, Zimmermann M, Marquardt G, Lanfermann H, Nafe R, Seifert V. Glioblastoma multiforme of the brain stem in a patient with acquired immunodeficiency syndrome. Acta Neurochir (Wien). 2002; 144:941–944. discussion 944-5.11. Hall JR, Short SC. Management of glioblastoma multiforme in HIV patients: a case series and review of published studies. Clin Oncol (R Coll Radiol). 2009; 21:591–597.
Article12. Gasnault J, Roux FX, Vedrenne C. Cerebral astrocytoma in association with HIV infection. J Neurol Neurosurg Psychiatry. 1988; 51:422–424.
Article13. Moulignier A, Mikol J, Pialoux G, Eliaszewicz M, Thurel C, Thiebaut JB. Cerebral glial tumors and human immunodeficiency virus-1 infection. More than a coincidental association. Cancer. 1994; 74:686–692.
Article14. Chamberlain MC. Gliomas in patients with acquired immune deficiency syndrome. Cancer. 1994; 74:1912–1914.
Article15. Gervasoni C, Ridolfo AL, Rocca A, Vago L, d’Arminio Monforte A. Cerebral astrocytoma in HIV-infected patients. AIDS. 1995; 9:403–404.
Article16. Neal JW, Llewelyn MB, Morrison HL, Jasani B, Borysiewicz LK. A malignant astrocytoma in a patient with AIDS: a possible association between astrocytomas and HIV infection. J Infect. 1996; 33:159–162.
Article17. Waubant E, Delisle MB, Bonafé A, Gray F, Clanet M. Cervical cord glioma in an HIV-positive patient. Eur Neurol. 1998; 39:58–60.18. Blumenthal DT, Raizer JJ, Rosenblum MK, Bilsky MH, Hariharan S, Abrey LE. Primary intracranial neoplasms in patients with HIV. Neurology. 1999; 52:1648–1651.
Article19. Vannemreddy PS, Fowler M, Polin RS, Todd JR, Nanda A. Glioblastoma multiforme in a case of acquired immunodeficiency syndrome: investigation a possible oncogenic influence of human immunodeficiency virus on glial cells. Case report and review of the literature. J Neurosurg. 2000; 92:161–164.
Article20. Simpson DM, Tagliati M. Neurologic manifestations of HIV infection. Ann Intern Med. 1994; 121:769–785.
Article21. Góngora-Rivera F, Santos-Zambrano J, Moreno-Andrade T, Calzada-López P, Soto-Hernández JL. The clinical spectrum of neurological manifestations in AIDS patients in Mexico. Arch Med Res. 2000; 31:393–398.
Article22. Petito CK, Cho ES, Lemann W, Navia BA, Price RW. Neuropathology of acquired immunodeficiency syndrome (AIDS): an autopsy review. J Neuropathol Exp Neurol. 1986; 45:635–646.23. Power C, Boissé L, Rourke S, Gill MJ. NeuroAIDS: an evolving epidemic. Can J Neurol Sci. 2009; 36:285–295.
Article24. Hajjar M, Lacoste D, Brossard G, et al. Non-acquired immune deficiency syndrome-defining malignancies in a hospital-based cohort of human immunodeficiency virus-infected patients: Bordeaux, France, 1985-1991. Groupe d'Epidémiologie Clinique du SIDA en Aquitaine. J Natl Cancer Inst. 1992; 84:1593–1595.
Article25. Tacconi L, Stapleton S, Signorelli F, Thomas DG. Acquired immune deficiency syndrome (AIDS) and cerebral astrocytoma. Clin Neurol Neurosurg. 1996; 98:149–151.
Article26. Ohgaki H, Dessen P, Jourde B, et al. Genetic pathways to glioblastoma: a population-based study. Cancer Res. 2004; 64:6892–6899.27. Wiley CA, Schrier RD, Nelson JA, Lampert PW, Oldstone MB. Cellular localization of human immunodeficiency virus infection within the brains of acquired immune deficiency syndrome patients. Proc Natl Acad Sci U S A. 1986; 83:7089–7093.
Article28. Brack-Werner R, Kleinschmidt A, Ludvigsen A, et al. Infection of human brain cells by HIV-1: restricted virus production in chronically infected human glial cell lines. AIDS. 1992; 6:273–285.29. Geleziunas R, Schipper HM, Wainberg MA. Pathogenesis and therapy of HIV-1 infection of the central nervous system. AIDS. 1992; 6:1411–1426.
Article30. Liu Y, Liu H, Kim BO, et al. CD4-independent infection of astrocytes by human immunodeficiency virus type 1: requirement for the human mannose receptor. J Virol. 2004; 78:4120–4133.
Article31. Carrana EJ, Rossitch E, Moore MR, Funkenstein HH. Anaplastic astrocytoma in association with human immunodeficiency virus type 1 infection. Am J Emerg Med. 1990; 8:565–567.
Article32. Vincendeau M, Kramer S, Hadian K, et al. Control of HIV replication in astrocytes by a family of highly conserved host proteins with a common Rev-interacting domain (Risp). AIDS. 2010; 24:2433–2442.
Article33. Felber BK, Zolotukhin AS, Pavlakis GN. Posttranscriptional control of HIV-1 and other retroviruses and its practical applications. Adv Pharmacol. 2007; 55:161–197.
Article34. Kjems J, Askjaer P. Rev protein and its cellular partners. Adv Pharmacol. 2000; 48:251–298.
Article35. Groom HC, Anderson EC, Dangerfield JA, Lever AM. Rev regulates translation of human immunodeficiency virus type 1 RNAs. J Gen Virol. 2009; 90(Pt 5):1141–1147.
Article36. Cusimano MD. An update on the cellular and molecular biology of brain tumors. Can J Neurol Sci. 1989; 16:22–27.
Article37. Kim CM, Vogel J, Jay G, Rhim JS. The HIV tat gene transforms human keratinocytes. Oncogene. 1992; 7:1525–1529.38. Vogel J, Hinrichs SH, Reynolds RK, Luciw PA, Jay G. The HIV tat gene induces dermal lesions resembling Kaposi's sarcoma in transgenic mice. Nature. 1988; 335:606–611.
Article39. Kramer-Hämmerle S, Kohleisen B, Hohenadl C, et al. HIV type 1 Nef promotes neoplastic transformation of immortalized neural cells. AIDS Res Hum Retroviruses. 2001; 17:597–602.
Article40. Briggs SD, Sharkey M, Stevenson M, Smithgall TE. SH3-mediated Hck tyrosine kinase activation and fibroblast transformation by the Nef protein of HIV-1. J Biol Chem. 1997; 272:17899–17902.
Article41. Du Z, Lang SM, Sasseville VG, et al. Identification of a nef allele that causes lymphocyte activation and acute disease in macaque monkeys. Cell. 1995; 82:665–674.
Article42. Kohleisen B, Shumay E, Sutter G, et al. Stable expression of HIV-1 Nef induces changes in growth properties and activation state of human astrocytes. AIDS. 1999; 13:2331–2341.
Article43. Selmaj KW, Farooq M, Norton WT, Raine CS, Brosnan CF. Proliferation of astrocytes in vitro in response to cytokines. A primary role for tumor necrosis factor. J Immunol. 1990; 144:129–135.44. Wiemels JL, Wiencke JK, Sison JD, Miike R, McMillan A, Wrensch M. History of allergies among adults with glioma and controls. Int J Cancer. 2002; 98:609–615.
Article45. Wiemels JL, Wilson D, Patil C, et al. IgE, allergy, and risk of glioma: update from the San Francisco Bay Area Adult Glioma Study in the temozolomide era. Int J Cancer. 2009; 125:680–687.
Article46. Salvati M, Frati A, Caroli E, et al. Glioblastoma in kidney transplant recipients. Report of five cases. J Neurooncol. 2003; 63:33–37.47. Basić-Jukić N, Basić-Kes V, Kes P, Furić-Cunko V, Bacić-Baronica K. [Neurological complications in renal transplant recipients]. Acta Med Croatica. 2008; 62:Suppl 1. 76–81.48. Schiff D, O’Neill B, Wijdicks E, Antin JH, Wen PY. Gliomas arising in organ transplant recipients: an unrecognized complication of transplantation? Neurology. 2001; 57:1486–1488.
Article49. Hiesse C, Kriaa F, Rieu P, et al. Incidence and type of malignancies occurring after renal transplantation in conventionally and cyclosporine-treated recipients: analysis of a 20-year period in 1600 patients. Transplant Proc. 1995; 27:972–974.50. Wahl SM, Allen JB, McCartney-Francis N, et al. Macrophage- and astrocyte-derived transforming growth factor beta as a mediator of central nervous system dysfunction in acquired immune deficiency syndrome. J Exp Med. 1991; 173:981–991.
Article51. Prins RM, Liau LM. Immunology and immunotherapy in neurosurgical disease. Neurosurgery. 2003; 53:144–152. discussion 152-3.
Article52. Barker CF, Billingham RE. Immunologically privileged sites. Adv Immunol. 1977; 25:1–54.
Article53. Walker PR, Calzascia T, Dietrich PY. All in the head: obstacles for immune rejection of brain tumours. Immunology. 2002; 107:28–38.
Article54. Walker PR, Calzascia T, Schnuriger V, et al. The brain parenchyma is permissive for full antitumor CTL effector function, even in the absence of CD4 T cells. J Immunol. 2000; 165:3128–3135.
Article55. Wiendl H, Mitsdoerffer M, Weller M. Hide-and-seek in the brain: a role for HLA-G mediating immune privilege for glioma cells. Semin Cancer Biol. 2003; 13:343–351.
Article56. Wiendl H, Mitsdoerffer M, Hofmeister V, et al. A functional role of HLA-G expression in human gliomas: an alternative strategy of immune escape. J Immunol. 2002; 168:4772–4780.
Article57. Dunn GP, Dunn IF, Curry WT. Focus on TILs: prognostic significance of tumor infiltrating lymphocytes in human glioma. Cancer Immun. 2007; 7:12.58. Liu G, Ying H, Zeng G, Wheeler CJ, Black KL, Yu JS. HER-2, gp100, and MAGE-1 are expressed in human glioblastoma and recognized by cytotoxic T cells. Cancer Res. 2004; 64:4980–4986.
Article59. Wu AH, Xiao J, Anker L, et al. Identification of EGFRvIII-derived CTL epitopes restricted by HLA A0201 for dendritic cell based immunotherapy of gliomas. J Neurooncol. 2006; 76:23–30.
Article60. Cserr HF, Knopf PM. Cervical lymphatics, the blood-brain barrier and the immunoreactivity of the brain: a new view. Immunol Today. 1992; 13:507–512.
Article61. Aloisi F, Ria F, Penna G, Adorini L. Microglia are more efficient than astrocytes in antigen processing and in Th1 but not Th2 cell activation. J Immunol. 1998; 160:4671–4680.62. Kushen MC, Sonabend AM, Lesniak MS. Current immunotherapeutic strategies for central nervous system tumors. Surg Oncol Clin N Am. 2007; 16:987–1004. xii
Article63. de Vos AF, van Meurs M, Brok HP, et al. Transfer of central nervous system autoantigens and presentation in secondary lymphoid organs. J Immunol. 2002; 169:5415–5423.
Article64. Yang I, Tihan T, Han SJ, et al. CD8+ T-cell infiltrate in newly diagnosed glioblastoma is associated with long-term survival. J Clin Neurosci. 2010; 17:1381–1385.
Article65. Struss AK, Romeike BF, Munnia A. PHF3-specific antibody responses in over 60% of patients with glioblastoma multiforme. Oncogene. 2001; 20:4107–4114.
Article66. Hickey WF, Hsu BL, Kimura H. T-lymphocyte entry into the central nervous system. J Neurosci Res. 1991; 28:254–260.
Article67. Calzascia T, Masson F, Di Berardino-Besson W, et al. Homing phenotypes of tumor-specific CD8 T cells are predetermined at the tumor site by crosspresenting APCs. Immunity. 2005; 22:175–184.
Article68. Vajkoczy P, Menger MD. Vascular microenvironment in gliomas. J Neurooncol. 2000; 50:99–108.
Article69. Perrin G, Schnuriger V, Quiquerez AL, et al. Astrocytoma infiltrating lymphocytes include major T cell clonal expansions confined to the CD8 subset. Int Immunol. 1999; 11:1337–1350.
Article70. Kennedy BC, Maier LM, D’Amico R, et al. Dynamics of central and peripheral immunomodulation in a murine glioma model. BMC Immunol. 2009; 10:11.
Article71. Platten M, Wick W, Weller M. Malignant glioma biology: role for TGF-beta in growth, motility, angiogenesis, and immune escape. Microsc Res Tech. 2001; 52:401–410.
Article72. Sinkovics JG, Horvath JC. Vaccination against human cancers (review). Int J Oncol. 2000; 16:81–96.
Article73. Palma L, Di Lorenzo N, Guidetti B. Lymphocytic infiltrates in primary glioblastomas and recidivous gliomas. Incidence, fate, and relevance to prognosis in 228 operated cases. J Neurosurg. 1978; 49:854–861.
Article74. Böker DK, Kalff R, Gullotta F, Weekes-Seifert S, Möhrer U. Mononuclear infiltrates in human intracranial tumors as a prognostic factor. Influence of preoperative steroid treatment. I. Glioblastoma. Clin Neuropathol. 1984; 3:143–147.75. Schiffer D, Cavicchioli D, Giordana MT, Palmucci L, Piazza A. Analysis of some factors effecting survival in malignant gliomas. Tumori. 1979; 65:119–125.
Article76. Rossi ML, Jones NR, Candy E, et al. The mononuclear cell infiltrate compared with survival in high-grade astrocytomas. Acta Neuropathol. 1989; 78:189–193.
Article77. Safdari H, Hochberg FH, Richardson EP Jr. Prognostic value of round cell (lymphocyte) infiltration in malignant gliomas. Surg Neurol. 1985; 23:221–226.
Article78. Palella FJ Jr, Baker RK, Moorman AC, et al. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr. 2006; 43:27–34.79. Powderly WG, Landay A, Lederman MM. Recovery of the immune system with antiretroviral therapy: the end of opportunism? JAMA. 1998; 280:72–77.
Article80. Guihot A, Bourgarit A, Carcelain G, Autran B. Immune reconstitution after a decade of combined antiretroviral therapies for human immunodeficiency virus. Trends Immunol. 2011; 32:131–137.
Article81. Corbeau P, Reynes J. Immune reconstitution under antiretroviral therapy: the new challenge in HIV-1 infection. Blood. 2011; 117:5582–5590.
Article82. Plettenberg A, Brockmeyer NH, Haastert B, et al. Impact of earlier HAART initiation on the immune status and clinical course of treated patients on the basis of cohort data of the German Competence Network for HIV/AIDS. Infection. 2011; 39:3–12.
Article83. Harrington M, Carpenter CC. Hit HIV-1 hard, but only when necessary. Lancet. 2000; 355:2147–2152.
Article84. Jain V, Deeks SG. When to start antiretroviral therapy. Curr HIV/AIDS Rep. 2010; 7:60–68.
Article85. Leone S, Gregis G, Quinzan G, et al. Causes of death and risk factors among HIV-infected persons in the HAART era: analysis of a large urban cohort. Infection. 2011; 39:13–20.
Article86. Shelburne SA, Visnegarwala F, Darcourt J, et al. Incidence and risk factors for immune reconstitution inflammatory syndrome during highly active antiretroviral therapy. AIDS. 2005; 19:399–406.
Article87. Moyle G. Clinical manifestations and management of antiretroviral nucleoside analog-related mitochondrial toxicity. Clin Ther. 2000; 22:911–936. discussion 898.
Article88. Autran B, Carcelaint G, Li TS, et al. Restoration of the immune system with anti-retroviral therapy. Immunol Lett. 1999; 66:207–211.
Article89. Martin D. Immune restoration in the context of HAART. South Afr J HIV Med. 2004; 5:8–14.90. Wang J, Lin HS, Liu MY, Li Y. Immune reconstitution of acquired immune deficiency syndrome. Chin J Integr Med. 2010; 16:557–564.
Article91. Xia C, Luo D, Yu X, Jiang S, Liu S. HIV-associated dementia in the era of highly active antiretroviral therapy (HAART). Microbes Infect. 2011; 13:419–425.
Article92. Dal Maso L, Polesel J, Serraino D, et al. Pattern of cancer risk in persons with AIDS in Italy in the HAART era. Br J Cancer. 2009; 100:840–847.
Article93. Shiels MS, Cole SR, Kirk GD, Poole C. A meta-analysis of the incidence of non-AIDS cancers in HIV-infected individuals. J Acquir Immune Defic Syndr. 2009; 52:611–622.
Article94. Aboulafia DM, Puswella AL. Highly active antiretroviral therapy as the sole treatment for AIDS-related primary central nervous system lymphoma: a case report with implications for treatment. AIDS Patient Care STDS. 2007; 21:900–907.
Article95. Bayraktar S, Bayraktar UD, Ramos JC, Stefanovic A, Lossos IS. Primary CNS lymphoma in HIV positive and negative patients: comparison of clinical characteristics, outcome and prognostic factors. J Neurooncol. 2011; 101:257–265.
Article96. Laurent N, de Boüard S, Guillamo JS, et al. Effects of the proteasome inhibitor ritonavir on glioma growth in vitro and in vivo. Mol Cancer Ther. 2004; 3:129–136.97. Zundel W, Schindler C, Haas-Kogan D, et al. Loss of PTEN facilitates HIF-1-mediated gene expression. Genes Dev. 2000; 14:391–396.
Article98. Gupta AK, Cerniglia GJ, Mick R, McKenna WG, Muschel RJ. HIV protease inhibitors block Akt signaling and radiosensitize tumor cells both in vitro and in vivo. Cancer Res. 2005; 65:8256–8265.
Article99. Radu A, Neubauer V, Akagi T, Hanafusa H, Georgescu MM. PTEN induces cell cycle arrest by decreasing the level and nuclear localization of cyclin D1. Mol Cell Biol. 2003; 23:6139–6149.
Article100. Pore N, Jiang Z, Shu HK, Bernhard E, Kao GD, Maity A. Akt1 activation can augment hypoxia-inducible factor-1alpha expression by increasing protein translation through a mammalian target of rapamycin-independent pathway. Mol Cancer Res. 2006; 4:471–479.
Article101. Pore N, Liu S, Shu HK, et al. Sp1 is involved in Akt-mediated induction of VEGF expression through an HIF-1-independent mechanism. Mol Biol Cell. 2004; 15:4841–4853.
Article102. Zhong H, Chiles K, Feldser D, et al. Modulation of hypoxia-inducible factor 1alpha expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: implications for tumor angiogenesis and therapeutics. Cancer Res. 2000; 60:1541–1545.103. Pore N, Gupta AK, Cerniglia GJ, et al. Nelfinavir down-regulates hypoxia-inducible factor 1alpha and VEGF expression and increases tumor oxygenation: implications for radiotherapy. Cancer Res. 2006; 66:9252–9259.
Article104. Carmeliet P. VEGF as a key mediator of angiogenesis in cancer. Oncology. 2005; 69:Suppl 3. 4–10.
Article105. Pore N, Gupta AK, Cerniglia GJ, Maity A. HIV protease inhibitors decrease VEGF/HIF-1alpha expression and angiogenesis in glioblastoma cells. Neoplasia. 2006; 8:889–895.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Meningioma Compatible with Metastatic Glioblastoma Multiforme
- A Case of Multicentric Glioblastoma Multiforme
- A Case of Glioblastoma Multiforme of the Cerebellum
- Leser-Trelat Sign in Glioblastoma Multiforme
- Scalp Metastasis of Glioblastoma Multiforme after Craniotomy and stereotatic Interstitial Brachytherapy