Cancer Res Treat.
2016 Oct;48(4):1429-1437. 10.4143/crt.2015.464.
A Pilot Study Evaluating Steroid-Induced Diabetes after Antiemetic Dexamethasone Therapy in Chemotherapy-Treated Cancer Patients
- Affiliations
-
- 1Department of Internal Medicine, Chungbuk National University College of Medicine, Cheongju, Korea. sook3529@hanmail.net
- KMID: 2356245
- DOI: http://doi.org/10.4143/crt.2015.464
Abstract
- PURPOSE
Dexamethasone is a mainstay antiemetic regimen for the prevention of chemotherapy-induced nausea and vomiting. The aim of this pilot study was to assess the incidence of and factors associated with steroid-induced diabetes in cancer patients receiving chemotherapy with dexamethasone as an antiemetic.
MATERIALS AND METHODS
Non-diabetic patients with newly diagnosed gastrointestinal cancer who received at least three cycles of highly or moderately emetogenic chemotherapy with dexamethasone as an antiemetic were enrolled. Fasting plasma glucose levels, 2-hour postprandial glucose levels, and hemoglobin A1C tests for the diagnosis of diabetes were performed before chemotherapy and at 3 and 6 months after the start of chemotherapy. The homeostasis model assessment of insulin resistance (HOMA-IR) was used as an index for measurement of insulin resistance, defined as a HOMA-IR ≥ 2.5.
RESULTS
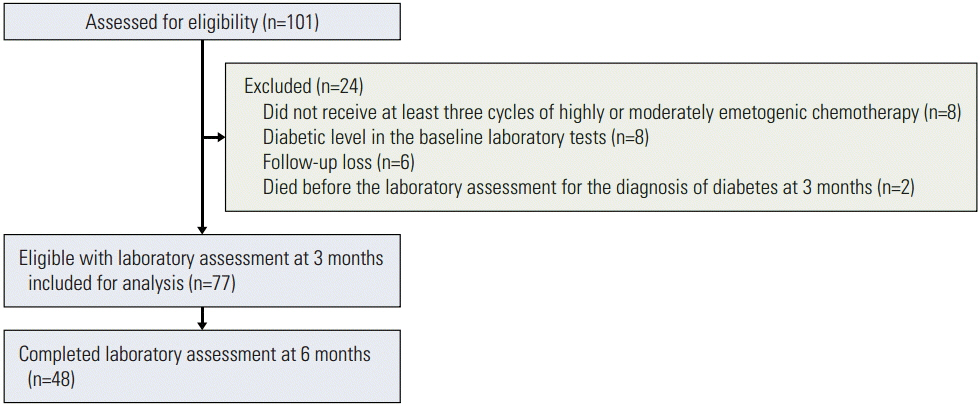
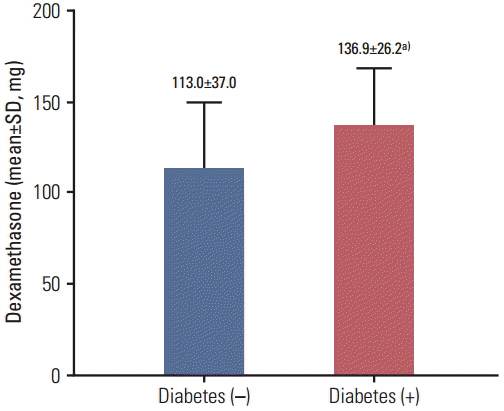
Between January 2012 and November 2013, 101 patients with no history of diabetes underwent laboratory tests for assessment of eligibility; 77 of these patients were included in the analysis. Forty-five patients (58.4%) were insulin resistant and 17 (22.1%) developed steroid-induced diabetes at 3 or 6 months after the first chemotherapy, which included dexamethasone as an antiemetic. Multivariate analysis showed significant association of the incidence of steroid-induced diabetes with the cumulative dose of dexamethasone (p=0.049).
CONCLUSION
We suggest that development of steroid-induced diabetes after antiemetic dexamethasone therapy occurs in approximately 20% of non-diabetic cancer patients; this is particularly significant for patients receiving high doses of dexamethasone.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Vigneri P, Frasca F, Sciacca L, Pandini G, Vigneri R. Diabetes and cancer. Endocr Relat Cancer. 2009; 16:1103–23.
Article2. Oyer DS, Shah A, Bettenhausen S. How to manage steroid diabetes in the patient with cancer. J Support Oncol. 2006; 4:479–83.3. Poulson J. The management of diabetes in patients with advanced cancer. J Pain Symptom Manage. 1997; 13:339–46.
Article4. Hesketh PJ. Chemotherapy-induced nausea and vomiting. N Engl J Med. 2008; 358:2482–94.
Article5. NCCN Clinical Practice Guidelines in Oncology: antiemesis. Version 2, 2014 [Internet]. Fort Washington, PA: National Comprehensive Cancer Network;2014 [cited 2015 Mar 1]. Available from: http://www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf.6. Vardy J, Chiew KS, Galica J, Pond GR, Tannock IF. Side effects associated with the use of dexamethasone for prophylaxis of delayed emesis after moderately emetogenic chemotherapy. Br J Cancer. 2006; 94:1011–5.
Article7. Fardet L, Kassar A, Cabane J, Flahault A. Corticosteroid-induced adverse events in adults: frequency, screening and prevention. Drug Saf. 2007; 30:861–81.8. Han HS, Park JC, Park SY, Lee KT, Bae SB, Kim HJ, et al. A prospective multicenter study evaluating secondary adrenal suppression after antiemetic dexamethasone therapy in ccancer patients receiving chemotherapy: a Korean South West Oncology Group Study. Oncologist. 2015; 20:1432–9.9. Rafacho A, Ortsater H, Nadal A, Quesada I. Glucocorticoid treatment and endocrine pancreas function: implications for glucose homeostasis, insulin resistance and diabetes. J Endocrinol. 2014; 223:R49–62.
Article10. Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson JL, Loscalzo J. Harrison’s principles of internal medicine. 18th ed. New York: McGraw-Hill Companies, Inc.;2011.11. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985; 28:412–9.12. Kim CH, Kim HK, Kim EH, Bae SJ, Park JY. Relative contributions of insulin resistance and beta-cell dysfunction to the development of Type 2 diabetes in Koreans. Diabet Med. 2013; 30:1075–9.13. Son JW, Park CY, Kim S, Lee HK, Lee YS; Insulin Resistance as Primary Pathogenesis in Newly Diagnosed, Drug Naïve Type 2 Diabetes Patients in Korea (SURPRISE) Study Group. Changing clinical characteristics according to insulin resistance and insulin secretion in newly diagnosed type 2 diabetic patients in Korea. Diabetes Metab J. 2015; 39:387–94.
Article14. Yoo KE, Kang RY, Lee JY, Lee YJ, Suh SY, Kim KS, et al. Awareness of the adverse effects associated with prophylactic corticosteroid use during docetaxel therapy. Support Care Cancer. 2015; 23:1969–77.
Article15. Brunello A, Kapoor R, Extermann M. Hyperglycemia during chemotherapy for hematologic and solid tumors is correlated with increased toxicity. Am J Clin Oncol. 2011; 34:292–6.
Article16. Polonsky KS, Rubenstein AH. C-peptide as a measure of the secretion and hepatic extraction of insulin. Pitfalls and limitations. Diabetes. 1984; 33:486–94.
Article17. Weiser MA, Cabanillas ME, Konopleva M, Thomas DA, Pierce SA, Escalante CP, et al. Relation between the duration of remission and hyperglycemia during induction chemotherapy for acute lymphocytic leukemia with a hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone/methotrexate-cytarabine regimen. Cancer. 2004; 100:1179–85.
Article18. Meyerhardt JA, Catalano PJ, Haller DG, Mayer RJ, Macdonald JS, Benson AB 3rd, et al. Impact of diabetes mellitus on outcomes in patients with colon cancer. J Clin Oncol. 2003; 21:433–40.
Article19. van de Poll-Franse LV, Houterman S, Janssen-Heijnen ML, Dercksen MW, Coebergh JW, Haak HR. Less aggressive treatment and worse overall survival in cancer patients with diabetes: a large population based analysis. Int J Cancer. 2007; 120:1986–92.
Article20. Barone BB, Yeh HC, Snyder CF, Peairs KS, Stein KB, Derr RL, et al. Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: a systematic review and meta-analysis. JAMA. 2008; 300:2754–64.21. Yancik R, Wesley MN, Ries LA, Havlik RJ, Edwards BK, Yates JW. Effect of age and comorbidity in postmenopausal breast cancer patients aged 55 years and older. JAMA. 2001; 285:885–92.
Article22. Luo J, Chen YJ, Chang LJ. Fasting blood glucose level and prognosis in non-small cell lung cancer (NSCLC) patients. Lung Cancer. 2012; 76:242–7.
Article23. Nakazawa K, Kurishima K, Tamura T, Ishikawa H, Satoh H, Hizawa N. Survival difference in NSCLC and SCLC patients with diabetes mellitus according to the first-line therapy. Med Oncol. 2013; 30:367.
Article24. Tran TT, Naigamwalla D, Oprescu AI, Lam L, McKeown-Eyssen G, Bruce WR, et al. Hyperinsulinemia, but not other factors associated with insulin resistance, acutely enhances colorectal epithelial proliferation in vivo. Endocrinology. 2006; 147:1830–7.
Article25. Muller LM, Gorter KJ, Hak E, Goudzwaard WL, Schellevis FG, Hoepelman AI, et al. Increased risk of common infections in patients with type 1 and type 2 diabetes mellitus. Clin Infect Dis. 2005; 41:281–8.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Granisetron Plus Dexamethasone in the Prevention of Delayed Nausea and Vomiting
- Antiemetic Effect of Granisetron plus Dexamethasone for the Patients Refractory to Metoclopramide , Dexamethasone and Lorazepam ( MDL )
- A Comparison of the Acute Antiemetic Effect of Tropisetron with Ondansetron in Patients Receiving Cisplatin
- Palonosetron versus granisetron in combination with aprepitant for the prevention of chemotherapy-induced nausea and vomiting in patients with gynecologic cancer
- A comparison of the acute antiemetic effect of ondansetron with combination of metoclopramide, dexamethasone, lorazepam in patients receiving cisplatin