Cancer Res Treat.
2016 Oct;48(4):1373-1381. 10.4143/crt.2015.475.
Prognostic Value of Axillary Nodal Ratio after Neoadjuvant Chemotherapy of Doxorubicin/Cyclophosphamide Followed by Docetaxel in Breast Cancer: A Multicenter Retrospective Cohort Study
- Affiliations
-
- 1Division of Hematology and Medical Oncology, Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea. jhkimmd@snu.ac.kr
- 2Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 3Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea.
- 4Department of Internal Medicine, SMG-SNU Boramae Medical Center, Seoul, Korea.
- 5Department of Internal Medicine, Kyungpook National University Hospital, Daegu, Korea.
- 6Department of Internal Medicine, Kyung Hee University Medical Center, Seoul, Korea.
- 7Department of Internal Medicine, Ajou University Hospital, Suwon, Korea.
- 8Department of Internal Medicine, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 9Department of Internal Medicine, National Cancer Center, Goyang, Korea.
- 10Department of Internal Medicine, Korea University Anam Hospital, Seoul, Korea.
- 11Department of Internal Medicine, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- 12Department of Internal Medicine, Samsung Medical Center, Seoul, Korea.
- KMID: 2356239
- DOI: http://doi.org/10.4143/crt.2015.475
Abstract
- PURPOSE
The purpose of this study is to investigate the prognostic value of lymph node (LN) ratio (LNR) in patients with breast cancer after neoadjuvant chemotherapy.
MATERIALS AND METHODS
This retrospective analysis is based on the data of 814 patientswith stage II/III breast cancer treated with four cycles of doxorubicin/cyclophosphamide followed by four cycles of docetaxel before surgery. We evaluated the clinical significance of LNR (3 categories: low 0-0.20 vs. intermediate 0.21-0.65 vs. high 0.66-1.00) using a Cox proportional regression model.
RESULTS
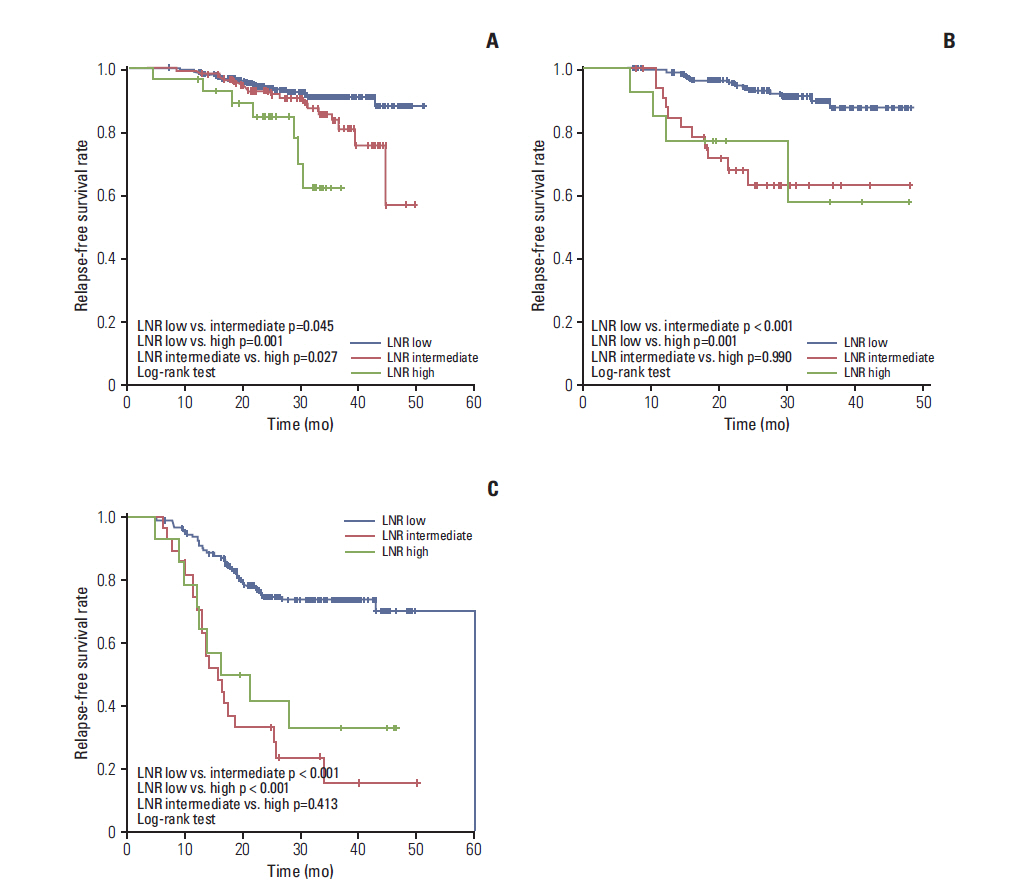
A total of 799 patients underwent breast surgery. Pathologic complete response (pCR, ypT0/isN0) was achieved in 129 patients (16.1%) (hormone receptor [HR] +/human epidermal growth factor receptor 2 [HER2] -, 34/373 [9.1%]; HER2+, 45/210 [21.4%]; triple negative breast cancer, 50/216 [23.1%]). The mean numbers of involved LN and retrieved LN were 2.70 (range, 0 to 42) and 13.98 (range, 1 to 64), respectively. The mean LNR was 0.17 (low, 574 [71.8%]; intermediate, 170 [21.3%]; high, 55 [6.9%]). In univariate analysis, LNR showed significant association with a worse relapse-free survival (3-year relapse-free survival rate 84.8% in low vs. 66.2% in intermediate vs. 54.3% in high; p < 0.001, log-rank test). In multivariate analysis, LNR did not show significant association with recurrence after adjusting for other clinical factors (age, histologic grade, subtype, ypT stage, ypN stage, lymphatic or vascular invasion, and pCR). In subgroup analysis, the LNR system had good prognostic value in HR+/HER2-subtype.
CONCLUSION
LNR is not superior to ypN stage in predicting clinical outcome of breast cancer after neoadjuvant chemotherapy. However, the prognostic value of the LNR system in HR+/HER2-patients is notable and worthy of further investigation.
MeSH Terms
Figure
Cited by 1 articles
-
Prognostic Significance of Inner Quadrant Involvement in Breast Cancer Treated with Neoadjuvant Chemotherapy
Ji Hyun Chang, Wan Jeon, Kyubo Kim, Kyung Hwan Shin, Wonshik Han, Dong-Young Noh, Seock-Ah Im, Tae-You Kim, Yung-Jue Bang
J Breast Cancer. 2016;19(4):394-401. doi: 10.4048/jbc.2016.19.4.394.
Reference
-
References
1. Fisher B, Bryant J, Wolmark N, Mamounas E, Brown A, Fisher ER, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 1998; 16:2672–85.
Article2. Bear HD, Anderson S, Smith RE, Geyer CE Jr, Mamounas EP, Fisher B, et al. Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer: National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2006; 24:2019–27.
Article3. van der Hage JA, van de Velde CJ, Julien JP, Tubiana-Hulin M, Vandervelden C, Duchateau L. Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. J Clin Oncol. 2001; 19:4224–37.
Article4. Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014; 384:164–72.
Article5. Kuerer HM, Newman LA, Buzdar AU, Hunt KK, Dhingra K, Buchholz TA, et al. Residual metastatic axillary lymph nodes following neoadjuvant chemotherapy predict disease-free survival in patients with locally advanced breast cancer. Am J Surg. 1998; 176:502–9.
Article6. Pierga JY, Mouret E, Dieras V, Laurence V, Beuzeboc P, Dorval T, et al. Prognostic value of persistent node involvement after neoadjuvant chemotherapy in patients with operable breast cancer. Br J Cancer. 2000; 83:1480–7.
Article7. Symmans WF, Peintinger F, Hatzis C, Rajan R, Kuerer H, Valero V, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol. 2007; 25:4414–22.
Article8. Colleoni M, Bagnardi V, Rotmensz N, Dellapasqua S, Viale G, Pruneri G, et al. A risk score to predict disease-free survival in patients not achieving a pathological complete remission after preoperative chemotherapy for breast cancer. Ann Oncol. 2009; 20:1178–84.
Article9. Neuman H, Carey LA, Ollila DW, Livasy C, Calvo BF, Meyer AA, et al. Axillary lymph node count is lower after neoadjuvant chemotherapy. Am J Surg. 2006; 191:827–9.
Article10. Erbes T, Orlowska-Volk M, Zur Hausen A, Rucker G, Mayer S, Voigt M, et al. Neoadjuvant chemotherapy in breast cancer significantly reduces number of yielded lymph nodes by axillary dissection. BMC Cancer. 2014; 14:4.
Article11. Woodward WA, Vinh-Hung V, Ueno NT, Cheng YC, Royce M, Tai P, et al. Prognostic value of nodal ratios in node-positive breast cancer. J Clin Oncol. 2006; 24:2910–6.
Article12. Vinh-Hung V, Verkooijen HM, Fioretta G, Neyroud-Caspar I, Rapiti E, Vlastos G, et al. Lymph node ratio as an alternative to pN staging in node-positive breast cancer. J Clin Oncol. 2009; 27:1062–8.
Article13. Danko ME, Bennett KM, Zhai J, Marks JR, Olson JA Jr. Improved staging in node-positive breast cancer patients using lymph node ratio: results in 1,788 patients with long-term follow-up. J Am Coll Surg. 2010; 210:797–805.
Article14. Ahn SH, Kim HJ, Lee JW, Gong GY, Noh DY, Yang JH, et al. Lymph node ratio and pN staging in patients with node-positive breast cancer: a report from the Korean breast cancer society. Breast Cancer Res Treat. 2011; 130:507–15.
Article15. Dings PJ, Elferink MA, Strobbe LJ, de Wilt JH. The prognostic value of lymph node ratio in node-positive breast cancer: a Dutch nationwide population-based study. Ann Surg Oncol. 2013; 20:2607–14.
Article16. Liu D, Chen Y, Deng M, Xie G, Wang J, Zhang L, et al. Lymph node ratio and breast cancer prognosis: a meta-analysis. Breast Cancer. 2014; 21:1–9.
Article17. Keam B, Im SA, Kim HJ, Oh DY, Kim JH, Lee SH, et al. Clinical significance of axillary nodal ratio in stage II/III breast cancer treated with neoadjuvant chemotherapy. Breast Cancer Res Treat. 2009; 116:153–60.
Article18. Saxena N, Hartman M, Aziz R, Rapiti E, Bhoo Pathy N, Lim SE, et al. Prognostic value of axillary lymph node status after neoadjuvant chemotherapy: results from a multicentre study. Eur J Cancer. 2011; 47:1186–92.
Article19. Chen S, Liu Y, Huang L, Chen CM, Wu J, Shao ZM. Lymph node counts and ratio in axillary dissections following neoadjuvant chemotherapy for breast cancer: a better alternative to traditional pN staging. Ann Surg Oncol. 2014; 21:42–50.
Article20. von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012; 30:1796–804.
Article21. Lips EH, Mulder L, de Ronde JJ, Mandjes IA, Koolen BB, Wessels LF, et al. Breast cancer subtyping by immunohistochemistry and histological grade outperforms breast cancer intrinsic subtypes in predicting neoadjuvant chemotherapy response. Breast Cancer Res Treat. 2013; 140:63–71.
Article22. Tausch C, Taucher S, Dubsky P, Seifert M, Reitsamer R, Kwasny W, et al. Prognostic value of number of removed lymph nodes, number of involved lymph nodes, and lymph node ratio in 7502 breast cancer patients enrolled onto trials of the Austrian Breast and Colorectal Cancer Study Group (ABCSG). Ann Surg Oncol. 2012; 19:1808–17.
Article23. Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ, et al. Strategies for subtypes: dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011; 22:1736–47.24. Sestak I, Dowsett M, Zabaglo L, Lopez-Knowles E, Ferree S, Cowens JW, et al. Factors predicting late recurrence for estrogen receptor-positive breast cancer. J Natl Cancer Inst. 2013; 105:1504–11.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Sentinel Lymph Node Biopsy in Patients with Clinically Negative Lymph Node After Neoadjuvant Chemotherapy
- Incidence of Febrile Neutropenia in Korean Female Breast Cancer Patients Receiving Preoperative or Postoperative Doxorubicin/Cyclophosphamide Followed by Docetaxel Chemotherapy
- Neoadjuvant Chemotherapy with Docetaxel and Adriamycin in Breast Cancer; Clincopathologic Factors Influencing to Response Rate
- Histomorphological Factors Predicting the Response to Neoadjuvant Chemotherapy in Triple-Negative Breast Cancer
- The Radiological Response Rate Pattern Is Associated With Recurrence Free Survival in Breast Cancer Patients Undergoing Neoadjuvant Chemotherapy