Asian Consensus Guidelines for the Diagnosis and Management of Gastrointestinal Stromal Tumor
- Affiliations
-
- 1Department of Internal Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 2Korean GIST Study Group, Seoul, Korea. ykkang@amc.seoul.kr
- 3Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 4Department of Pathology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 5Department of Surgery, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea.
- 6Department of Gastroenterology, Japanese Red Cross Nagoya Daini Hospital, Nagoya, Japan.
- 7Japanese GIST Subcommittee, Nishinomiya, Japan. tnishida@east.ncc.go.jp
- 8Department of Pathology, Hyogo College of Medicine, Nishinomiya, Japan.
- 9Department of Pathology, Peking University Third Hospital, Beijing, China.
- 10Chinese Expert Committee on GIST, Sichuan, China. lin100@medmail.com.cn
- 11Department of Gastrointestinal Surgery, West China Hospital, Sichuan University, Sichuan, China.
- 12Department of Pathology and Laboratory Medicine, Cathay General Hospital, Taipei, Taiwan.
- 13Department of Surgery, Chang Gung Memorial Hospital and University, Taoyuan, Taiwan.
- 14Department of Surgery, National Cancer Center Hospital East, Chiba, Japan.
- 15Department of Gastrointestinal Oncology, Peking University Cancer Hospital & Institute, Beijing, China.
- 16National Institute of Cancer Research, National Health Research Institutes, Tainan, Taiwan. leochen@nhri.org.tw
- 17Department of Internal Medicine, National Cheng Kung University Hospital, National Cheng Kung University, Tainan, Taiwan.
- KMID: 2356218
- DOI: http://doi.org/10.4143/crt.2016.187
Abstract
- Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors originating in the gastrointestinal tract. With the introduction of molecular-targeted therapy for GISTs which has yielded remarkable outcomes, these tumors have become a model of multidisciplinary oncological treatment. Although Western clinical guidelines are available for GISTs, such as those published by the National Comprehensive Cancer Network (NCCN) and the European Society of Medical Oncology (ESMO), the clinical situations in Asian countries are different from those in Western countries in terms of diagnostic methods, surgical approach, and availability of new targeted agents. Accordingly, we have reviewed current versions of several GIST guidelines published by Asian countries (Japan, Korea, China, and Taiwan) and the NCCN and ESMO and discussed the areas of dissensus. We here present the first version of the Asian GIST consensus guidelines that were prepared through a series of meetings involving multidisciplinary experts in the four countries. These guidelines provide an optimal approach to the diagnosis and management of GIST patients in Asian countries.
MeSH Terms
Figure
Cited by 4 articles
-
직장에 발생한 거대한 위장관 기질종양의 성공적인 내시경 절제
Seong Jung Kim, Yun Jung, Ran Hong, Jun Lee
Korean J Gastroenterol. 2021;78(4):235-239. doi: 10.4166/kjg.2021.097.젊은 환자에서 발생한 거대 악성 위장관 기질종양
Yun Jin Jeong, Hyun Seung Hwang, Yong Eun Park, Kyung Han Nam, Sung Jin Oh
Korean J Gastroenterol. 2022;79(4):177-181. doi: 10.4166/kjg.2022.006.Adjuvant Imatinib Treatment for 5 Years versus 3 Years in Patients with Ruptured Localized Gastrointestinal Stromal Tumor: A Retrospective Analysis
Sora Kang, Min-Hee Ryu, Yeong Hak Bang, Hyung-Don Kim, Hyung Eun Lee, Yoon-Koo Kang
Cancer Res Treat. 2022;54(4):1167-1174. doi: 10.4143/crt.2021.1040.Gastrointestinal Stromal Tumor of Small Intestine
Hyo Yeop Song, Geom Seog Seo, Hoon Gil Jo
Korean J Gastroenterol. 2023;81(6):276-278. doi: 10.4166/kjg.2023.049.
Reference
-
References
1. Corless CL, Barnett CM, Heinrich MC. Gastrointestinal stromal tumours: origin and molecular oncology. Nat Rev Cancer. 2011; 11:865–78.
Article2. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: soft tissue sarcoma, version 1 [Internet]. Washington, PA: National Comprehensive Cancer Network;2015. [cited 2015 Jun 15]. Available from: http://www.nccn.org/professionals/physician_gls/pdf/sarcoma.pdf.3. ESMO/European Sarcoma Network Working Group. Gastrointestinal stromal tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014; 25 Suppl 3:iii21–6.4. Kang YK, Kang HJ, Kim KM, Sohn T, Choi D, Ryu MH, et al. Clinical practice guideline for accurate diagnosis and effective treatment of gastrointestinal stromal tumor in Korea. Cancer Res Treat. 2012; 44:85–96.
Article5. Nishida T, Hirota S, Yanagisawa A, Sugino Y, Minami M, Yamamura Y, et al. Clinical practice guidelines for gastrointestinal stromal tumor (GIST) in Japan: English version. Int J Clin Oncol. 2008; 13:416–30.
Article6. Expert Committee on Gastrointestinal Stromal Tumors, CSCO. China consensus on diagnosis and treatment of gastrointestinal stromal tumors (2013). Chin Clin Oncol. 2013; 18:1025–34.7. Yeh CN, Hwang TL, Huang CS, Lee PH, Wu CW, Chen-Guo K, et al. Clinical practice guidelines for patients with gastrointestinal stromal tumor in Taiwan. World J Surg Oncol. 2012; 10:246.
Article8. Kim KM, Kang DW, Moon WS, Park JB, Park CK, Sohn JH, et al. Gastrointestinal stromal tumors in Koreans: it's incidence and the clinical, pathologic and immunohistochemical findings. J Korean Med Sci. 2005; 20:977–84.
Article9. Demetri GD, von Mehren M, Antonescu CR, DeMatteo RP, Ganjoo KN, Maki RG, et al. NCCN Task Force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw. 2010; 8 Suppl 2:S1–41.
Article10. Heinrich MC, Corless CL, Demetri GD, Blanke CD, von Mehren M, Joensuu H, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol. 2003; 21:4342–9.
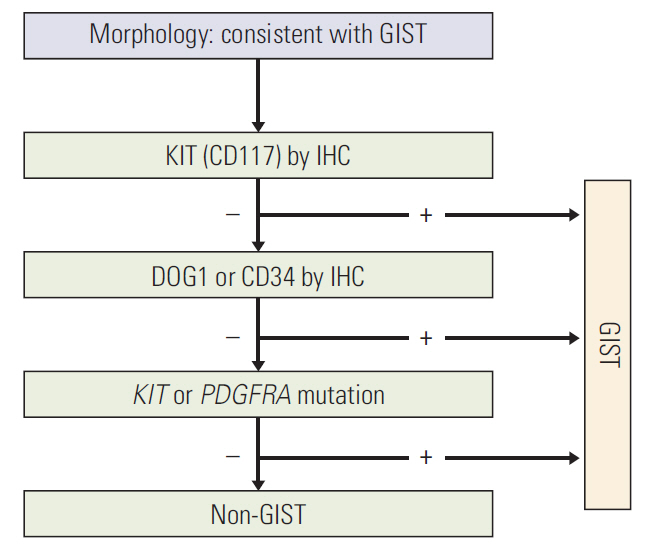
Article11. Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol. 2002; 33:459–65.
Article12. Liegl B, Hornick JL, Corless CL, Fletcher CD. Monoclonal antibody DOG1.1 shows higher sensitivity than KIT in the diagnosis of gastrointestinal stromal tumors, including unusual subtypes. Am J Surg Pathol. 2009; 33:437–46.
Article13. Kang GH, Srivastava A, Kim YE, Park HJ, Park CK, Sohn TS, et al. DOG1 and PKC-theta are useful in the diagnosis of KIT-negative gastrointestinal stromal tumors. Mod Pathol. 2011; 24:866–75.14. Kim KM, Kang DW, Moon WS, Park JB, Park CK, Sohn JH, et al. PKCtheta expression in gastrointestinal stromal tumor. Mod Pathol. 2006; 19:1480–6.15. Debiec-Rychter M, Sciot R, Le Cesne A, Schlemmer M, Hohenberger P, van Oosterom AT, et al. KIT mutations and dose selection for imatinib in patients with advanced gastrointestinal stromal tumours. Eur J Cancer. 2006; 42:1093–103.16. Gao J, Dang Y, Sun N, Li J, Shen L. C-KIT mutations were closely associated with the response to Imatinib in Chinese advanced gastrointestinal stromal tumor patients. Med Oncol. 2012; 29:3039–45.17. Kang HJ, Ryu MH, Kim KM, Park YS, Choi J, Ryoo BY, et al. Imatinib efficacy by tumor genotype in Korean patients with advanced gastrointestinal stromal tumors (GIST): The Korean GIST Study Group (KGSG) study. Acta Oncol. 2012; 51:528–36.
Article18. Miranda C, Nucifora M, Molinari F, Conca E, Anania MC, Bordoni A, et al. KRAS and BRAF mutations predict primary resistance to imatinib in gastrointestinal stromal tumors. Clin Cancer Res. 2012; 18:1769–76.
Article19. Oudijk L, Gaal J, Korpershoek E, van Nederveen FH, Kelly L, Schiavon G, et al. SDHA mutations in adult and pediatric wild-type gastrointestinal stromal tumors. Mod Pathol. 2013; 26:456–63.
Article20. Janeway KA, Kim SY, Lodish M, Nose V, Rustin P, Gaal J, et al. Defects in succinate dehydrogenase in gastrointestinal stromal tumors lacking KIT and PDGFRA mutations. Proc Natl Acad Sci U S A. 2011; 108:314–8.21. Miettinen M, Wang ZF, Sarlomo-Rikala M, Osuch C, Rutkowski P, Lasota J. Succinate dehydrogenase-deficient GISTs: a clinicopathologic, immunohistochemical, and molecular genetic study of 66 gastric GISTs with predilection to young age. Am J Surg Pathol. 2011; 35:1712–21.22. Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol. 2006; 23:70–83.
Article23. Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol. 2008; 39:1411–9.
Article24. Rutkowski P, Bylina E, Wozniak A, Nowecki ZI, Osuch C, Matlok M, et al. Validation of the Joensuu risk criteria for primary resectable gastrointestinal stromal tumour: the impact of tumour rupture on patient outcomes. Eur J Surg Oncol. 2011; 37:890–6.25. Joensuu H, Vehtari A, Riihimaki J, Nishida T, Steigen SE, Brabec P, et al. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol. 2012; 13:265–74.
Article26. Yanagimoto Y, Takahashi T, Muguruma K, Toyokawa T, Kusanagi H, Omori T, et al. Re-appraisal of risk classifications for primary gastrointestinal stromal tumors (GISTs) after complete resection: indications for adjuvant therapy. Gastric Cancer. 2015; 18:426–33.
Article27. Blay JY, Bonvalot S, Casali P, Choi H, Debiec-Richter M, Dei Tos AP, et al. Consensus meeting for the management of gastrointestinal stromal tumors: report of the GIST Consensus Conference of 20-21 March 2004, under the auspices of ESMO. Ann Oncol. 2005; 16:566–78.
Article28. Ryu MH, Lee JL, Chang HM, Kim TW, Kang HJ, Sohn HJ, et al. Patterns of progression in gastrointestinal stromal tumor treated with imatinib mesylate. Jpn J Clin Oncol. 2006; 36:17–24.
Article29. Gold JS, Dematteo RP. Combined surgical and molecular therapy: the gastrointestinal stromal tumor model. Ann Surg. 2006; 244:176–84.30. Dumonceau JM, Polkowski M, Larghi A, Vilmann P, Giovannini M, Frossard JL, et al. Indications, results, and clinical impact of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2011; 43:897–912.
Article31. Iwahashi M, Takifuji K, Ojima T, Nakamura M, Nakamori M, Nakatani Y, et al. Surgical management of small gastrointestinal stromal tumors of the stomach. World J Surg. 2006; 30:28–35.
Article32. McCarter MD, Antonescu CR, Ballman KV, Maki RG, Pisters PW, Demetri GD, et al. Microscopically positive margins for primary gastrointestinal stromal tumors: analysis of risk factors and tumor recurrence. J Am Coll Surg. 2012; 215:53–9.
Article33. Novitsky YW, Kercher KW, Sing RF, Heniford BT. Long-term outcomes of laparoscopic resection of gastric gastrointestinal stromal tumors. Ann Surg. 2006; 243:738–45.
Article34. Koh YX, Chok AY, Zheng HL, Tan CS, Chow PK, Wong WK, et al. A systematic review and meta-analysis comparing laparoscopic versus open gastric resections for gastrointestinal stromal tumors of the stomach. Ann Surg Oncol. 2013; 20:3549–60.
Article35. DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg. 2000; 231:51–8.36. Joensuu H, Reichardt P, Eriksson M, Sundby Hall K, Vehtari A. Gastrointestinal stromal tumor: a method for optimizing the timing of CT scans in the follow-up of cancer patients. Radiology. 2014; 271:96–103.
Article37. Desai J, Shankar S, Heinrich MC, Fletcher JA, Fletcher CD, Manola J, et al. Clonal evolution of resistance to imatinib in patients with metastatic gastrointestinal stromal tumors. Clin Cancer Res. 2007; 13(18 Pt 1):5398–405.
Article38. Raut CP, Posner M, Desai J, Morgan JA, George S, Zahrieh D, et al. Surgical management of advanced gastrointestinal stromal tumors after treatment with targeted systemic therapy using kinase inhibitors. J Clin Oncol. 2006; 24:2325–31.
Article39. Sym SJ, Ryu MH, Lee JL, Chang HM, Kim TW, Kim HC, et al. Surgical intervention following imatinib treatment in patients with advanced gastrointestinal stromal tumors (GISTs). J Surg Oncol. 2008; 98:27–33.
Article40. Du CY, Zhou Y, Song C, Wang YP, Jie ZG, He YL, et al. Is there a role of surgery in patients with recurrent or metastatic gastrointestinal stromal tumours responding to imatinib: a prospective randomised trial in China. Eur J Cancer. 2014; 50:1772–8.
Article41. Park SJ, Ryu MH, Ryoo BY, Park YS, Sohn BS, Kim HJ, et al. The role of surgical resection following imatinib treatment in patients with recurrent or metastatic gastrointestinal stromal tumors: results of propensity score analyses. Ann Surg Oncol. 2014; 21:4211–7.
Article42. An HJ, Ryu MH, Ryoo BY, Sohn BS, Kim KH, Oh ST, et al. The effects of surgical cytoreduction prior to imatinib therapy on the prognosis of patients with advanced GIST. Ann Surg Oncol. 2013; 20:4212–8.
Article43. Dematteo RP, Ballman KV, Antonescu CR, Maki RG, Pisters PW, Demetri GD, et al. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet. 2009; 373:1097–104.
Article44. Li J, Gong JF, Wu AW, Shen L. Post-operative imatinib in patients with intermediate or high risk gastrointestinal stromal tumor. Eur J Surg Oncol. 2011; 37:319–24.
Article45. Joensuu H, Eriksson M, Sundby Hall K, Hartmann JT, Pink D, Schutte J, et al. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA. 2012; 307:1265–72.46. Agaimy A, Vassos N, Croner RS. Gastrointestinal manifestations of neurofibromatosis type 1 (Recklinghausen's disease): clinicopathological spectrum with pathogenetic considerations. Int J Clin Exp Pathol. 2012; 5:852–62.47. Bumming P, Andersson J, Meis-Kindblom JM, Klingenstierna H, Engstrom K, Stierner U, et al. Neoadjuvant, adjuvant and palliative treatment of gastrointestinal stromal tumours (GIST) with imatinib: a centre-based study of 17 patients. Br J Cancer. 2003; 89:460–4.
Article48. Oh JS, Lee JL, Kim MJ, Ryu MH, Chang HM, Kim TW, et al. Neoadjuvant imatinib in locally advanced gastrointestinal stromal tumors of the stomach: report of three cases. Cancer Res Treat. 2006; 38:178–83.49. Prior JO, Montemurro M, Orcurto MV, Michielin O, Luthi F, Benhattar J, et al. Early prediction of response to sunitinib after imatinib failure by 18F-fluorodeoxyglucose positron emission tomography in patients with gastrointestinal stromal tumor. J Clin Oncol. 2009; 27:439–45.
Article50. Kim TW, Ryu MH, Lee H, Sym SJ, Lee JL, Chang HM, et al. Kinase mutations and efficacy of imatinib in Korean patients with advanced gastrointestinal stromal tumors. Oncologist. 2009; 14:540–7.
Article51. Yeh CN, Chen TW, Lee HL, Liu YY, Chao TC, Hwang TL, et al. Kinase mutations and imatinib mesylate response for 64 Taiwanese with advanced GIST: preliminary experience from Chang Gung Memorial Hospital. Ann Surg Oncol. 2007; 14:1123–8.
Article52. Imatinib dose escalation to 800 mg/day in Korean patients with metastatic or unresectable GIST harboring KIT exon 9 mutation (NCT01541709) [Internet]. ClinicalTrials.gov; 2015 [cited 2015 Jun 15]. Available from: http://clinicaltrials.gov/ct2/show/NCT01541709?term=NCT01541709&rank=1.53. Guilhot F. Indications for imatinib mesylate therapy and clinical management. Oncologist. 2004; 9:271–81.
Article54. Blay JY, Le Cesne A, Ray-Coquard I, Bui B, Duffaud F, Delbaldo C, et al. Prospective multicentric randomized phase III study of imatinib in patients with advanced gastrointestinal stromal tumors comparing interruption versus continuation of treatment beyond 1 year: the French Sarcoma Group. J Clin Oncol. 2007; 25:1107–13.
Article55. Lee JL, Ryu MH, Chang HM, Kim TW, Kang HJ, Sohn HJ, et al. Clinical outcome in gastrointestinal stromal tumor patients who interrupted imatinib after achieving stable disease or better response. Jpn J Clin Oncol. 2006; 36:704–11.
Article56. Le Cesne A, Ray-Coquard I, Bui BN, Adenis A, Rios M, Bertucci F, et al. Discontinuation of imatinib in patients with advanced gastrointestinal stromal tumours after 3 years of treatment: an open-label multicentre randomised phase 3 trial. Lancet Oncol. 2010; 11:942–9.
Article57. Choi H, Charnsangavej C, de Castro Faria S, Tamm EP, Benjamin RS, Johnson MM, et al. CT evaluation of the response of gastrointestinal stromal tumors after imatinib mesylate treatment: a quantitative analysis correlated with FDG PET findings. AJR Am J Roentgenol. 2004; 183:1619–28.58. Choi H, Charnsangavej C, Faria SC, Macapinlac HA, Burgess MA, Patel SR, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol. 2007; 25:1753–9.
Article59. Heinrich MC, Maki RG, Corless CL, Antonescu CR, Harlow A, Griffith D, et al. Primary and secondary kinase genotypes correlate with the biological and clinical activity of sunitinib in imatinib-resistant gastrointestinal stromal tumor. J Clin Oncol. 2008; 26:5352–9.
Article60. Yeh CN, Chen TW, Tseng JH, Liu YY, Wang SY, Tsai CY, et al. Surgical management in metastatic gastrointestinal stromal tumor (GIST) patients after imatinib mesylate treatment. J Surg Oncol. 2010; 102:599–603.
Article61. Zalcberg JR, Verweij J, Casali PG, Le Cesne A, Reichardt P, Blay JY, et al. Outcome of patients with advanced gastrointestinal stromal tumours crossing over to a daily imatinib dose of 800 mg after progression on 400 mg. Eur J Cancer. 2005; 41:1751–7.62. Park I, Ryu MH, Sym SJ, Lee SS, Jang G, Kim TW, et al. Dose escalation of imatinib after failure of standard dose in Korean patients with metastatic or unresectable gastrointestinal stromal tumor. Jpn J Clin Oncol. 2009; 39:105–10.
Article63. Yoo C, Ryu MH, Ryoo BY, Beck MY, Kang YK. Efficacy, safety, and pharmacokinetics of imatinib dose escalation to 800 mg/day in patients with advanced gastrointestinal stromal tumors. Invest New Drugs. 2013; 31:1367–74.64. Demetri GD, van Oosterom AT, Garrett CR, Blackstein ME, Shah MH, Verweij J, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006; 368:1329–38.
Article65. George S, Blay JY, Casali PG, Le Cesne A, Stephenson P, Deprimo SE, et al. Clinical evaluation of continuous daily dosing of sunitinib malate in patients with advanced gastrointestinal stromal tumour after imatinib failure. Eur J Cancer. 2009; 45:1959–68.
Article66. Li J, Gao J, Hong J, Shen L. Efficacy and safety of sunitinib in Chinese patients with imatinib-resistant or -intolerant gastrointestinal stromal tumors. Future Oncol. 2012; 8:617–24.
Article67. Chu TF, Rupnick MA, Kerkela R, Dallabrida SM, Zurakowski D, Nguyen L, et al. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet. 2007; 370:2011–9.
Article68. Torino F, Corsello SM, Longo R, Barnabei A, Gasparini G. Hypothyroidism related to tyrosine kinase inhibitors: an emerging toxic effect of targeted therapy. Nat Rev Clin Oncol. 2009; 6:219–28.
Article69. Demetri GD, Reichardt P, Kang YK, Blay JY, Rutkowski P, Gelderblom H, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013; 381:295–302.
Article70. Kang YK, Ryu MH, Yoo C, Ryoo BY, Kim HJ, Lee JJ, et al. Resumption of imatinib to control metastatic or unresectable gastrointestinal stromal tumours after failure of imatinib and sunitinib (RIGHT): a randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2013; 14:1175–82.
Article71. Sawaki A, Kanda T, Komatsu Y, Nishida T. Impact of rechallenge with imatinib in patients with advanced gastrointestinal stromal tumor after failure of imatinib and sunitinib. Gastroenterol Res Pract. 2014; 2014:342986.
Article72. Demetri GD, Wang Y, Wehrle E, Racine A, Nikolova Z, Blanke CD, et al. Imatinib plasma levels are correlated with clinical benefit in patients with unresectable/metastatic gastrointestinal stromal tumors. J Clin Oncol. 2009; 27:3141–7.
Article73. Eechoute K, Fransson MN, Reyners AK, de Jong FA, Sparreboom A, van der Graaf WT, et al. A long-term prospective population pharmacokinetic study on imatinib plasma concentrations in GIST patients. Clin Cancer Res. 2012; 18:5780–7.
Article74. Koo DH, Ryu MH, Ryoo BY, Beck MY, Na YS, Shin JG, et al. Association of ABCG2 polymorphism with clinical efficacy of imatinib in patients with gastrointestinal stromal tumor. Cancer Chemother Pharmacol. 2015; 75:173–82.
Article75. Yoon S, Ryu MH, Yoo C, Beck MY, Ryoo BY, Kang YK. Imatinib plasma monitoring-guided dose modification for managing imatinib-related toxicities in gastrointestinal stromal tumor patients. J Korean Med Sci. 2013; 28:1248–52.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Practice Guideline for Adequate Diagnosis and Effective Treatment of Gastrointestinal Stromal Tumor in Korea
- A High Risk Group in the Modified National Institutes of Health Consensus Criteria for the Gastrointestinal Stromal Tumor: A Clear Indication of the Adjuvant Imatinib
- A Case Report of a Bleeding Duodenal Gastro-Intestinal Stromal Tumor and its Emergent Management
- Incidental Gastrointestinal Subepithelial Mass
- Diagnosis of a Gastrointestinal Stromal Tumor Presenting as a Prostatic Mass: A Case Report