Restor Dent Endod.
2016 Nov;41(4):296-303. 10.5395/rde.2016.41.4.296.
Effects of proanthocyanidin, a crosslinking agent, on physical and biological properties of collagen hydrogel scaffold
- Affiliations
-
- 1Department of Conservative Dentistry, Wonkwang University Dental Hospital, Iksan, Korea.
- 2Department of Conservative Dentistry, School of Dentistry, Chonbuk National University, Jeonju, Korea. endomin@gmail.com
- 3Biomedical Research Institute of Chonbuk National University Hospital, Jeonju, Korea.
- KMID: 2356006
- DOI: http://doi.org/10.5395/rde.2016.41.4.296
Abstract
OBJECTIVES
The purpose of the present study was to evaluate the effects of proanthocyanidin (PAC), a crosslinking agent, on the physical properties of a collagen hydrogel and the behavior of human periodontal ligament cells (hPDLCs) cultured in the scaffold.
MATERIALS AND METHODS
Viability of hPDLCs treated with PAC was measured using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The physical properties of PAC treated collagen hydrogel scaffold were evaluated by the measurement of setting time, surface roughness, and differential scanning calorimetry (DSC). The behavior of the hPDLCs in the collagen scaffold was evaluated by cell morphology observation and cell numbers counting.
RESULTS
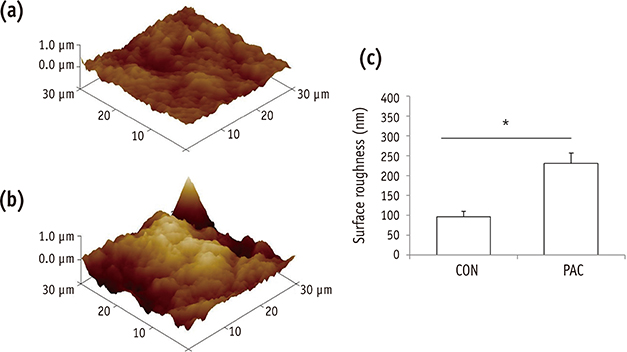
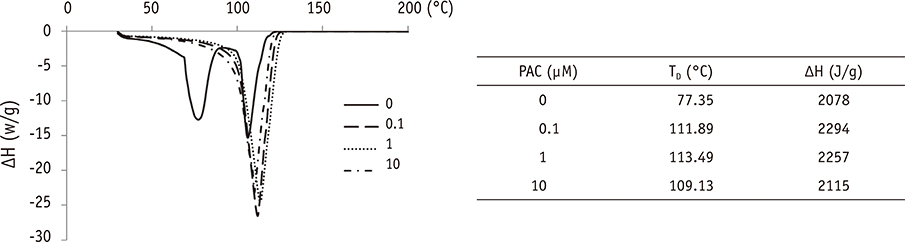
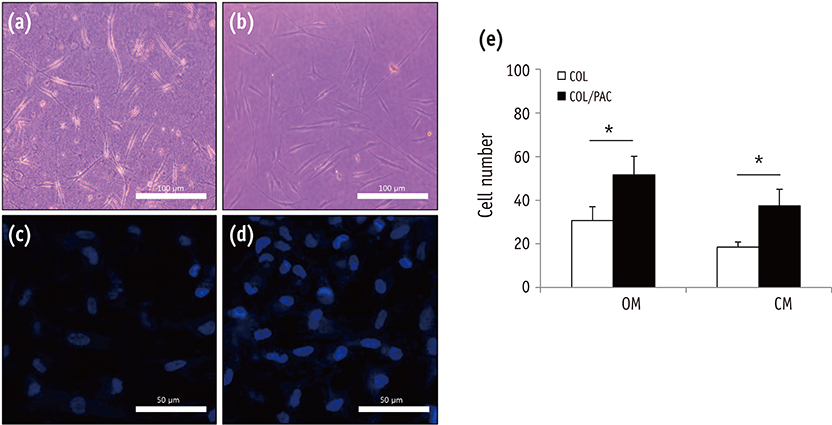
The setting time of the collagen scaffold was shortened in the presence of PAC (p < 0.05). The surface roughness of the PAC-treated collagen was higher compared to the untreated control group (p < 0.05). The thermogram of the crosslinked collagen exhibited a higher endothermic peak compared to the uncrosslinked one. Cells in the PAC-treated collagen were observed to attach in closer proximity to one another with more cytoplasmic extensions compared to cells in the untreated control group. The number of cells cultured in the PAC-treated collagen scaffolds was significantly increased compared to the untreated control (p < 0.05).
CONCLUSIONS
Our results showed that PAC enhanced the physical properties of the collagen scaffold. Furthermore, the proliferation of hPDLCs cultured in the collagen scaffold crosslinked with PAC was facilitated. Conclusively, the application of PAC to the collagen scaffold may be beneficial for engineering-based periodontal ligament regeneration in delayed replantation.
MeSH Terms
Figure
Reference
-
1. Krasner P, Rankow HJ. New philosophy for the treatment of avulsed teeth. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995; 79:616–623.
Article2. Hermann NV, Lauridsen E, Ahrensburg SS, Gerds TA, Andreasen JO. Periodontal healing complications following extrusive and lateral luxation in the permanent dentition: a longitudinal cohort study. Dent Traumatol. 2012; 28:394–402.
Article3. Andreasen JO, Borum MK, Jacobsen HL, Andreasen FM. Replantation of 400 avulsed permanent incisors. 4. Factors related to periodontal ligament healing. Endod Dent Traumatol. 1995; 11:76–89.
Article4. Mattioli-Belmonte M, Teti G, Salvatore V, Focaroli S, Orciani M, Dicarlo M, Fini M, Orsini G, Di Primio R, Falconi M. Stem cell origin differently affects bone tissue engineering strategies. Front Physiol. 2015; 6:266.
Article5. Wang Y, Cheung GS, Xu X, Zhao S, Zhang C. The effect of cultured autologous periodontal ligament cells on the healing of delayed autotransplanted dog's teeth. J Endod. 2010; 36:264–267.
Article6. Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, Young M, Robey PG, Wang CY, Shi S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004; 364:149–155.
Article7. Lekic P, Rojas J, Birek C, Tenenbaum H, McCulloch CA. Phenotypic comparison of periodontal ligament cells in vivo and in vitro. J Periodont Res. 2001; 36:71–79.
Article8. Yang ZH, Zhang XJ, Dang NN, Ma ZF, Xu L, Wu JJ, Sun YJ, Duan YZ, Lin Z, Jin Y. Apical tooth germ cell-conditioned medium enhances the differentiation of periodontal ligament stem cells into cementum/periodontal ligament-like tissues. J Periodontal Res. 2009; 44:199–210.
Article9. Huang GT, Gronthos S, Shi S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res. 2009; 88:792–806.
Article10. Catón J, Bostanci N, Remboutsika E, De Bari C, Mitsiadis TA. Future dentistry: cell therapy meets tooth and periodontal repair and regeneration. J Cell Mol Med. 2011; 15:1054–1065.
Article11. Han J, Menicanin D, Gronthos S, Bartold PM. Stem cells, tissue engineering and periodontal regeneration. Aust Dent J. 2014; 59:Supplement 1. 117–130.
Article12. Zhou Y, Li Y, Mao L, Peng H. Periodontal healing by periodontal ligament cell sheets in a teeth replantation model. Arch Oral Biol. 2012; 57:169–176.
Article13. Ivanovski S, Vaquette C, Gronthos S, Hutmacher DW, Bartold PM. Multiphasic scaffolds for periodontal tissue engineering. J Dent Res. 2014; 93:1212–1221.
Article14. Davidenko N, Schuster CF, Bax DV, Raynal N, Farndale RW, Best SM, Cameron RE. Control of crosslinking for tailoring collagen-based scaffolds stability and mechanics. Acta Biomater. 2015; 25:131–142.
Article15. Hutmacher DW, Cool S. Concepts of scaffold-based tissue engineering: the rationale to use solid free-form fabrication techniques. J Cell Mol Med. 2007; 11:654–669.
Article16. Wang Y, Van Manh N, Wang H, Zhong X, Zhang X, Li C. Synergistic intrafibrillar/extrafibrillar mineralization of collagen scaffolds based on a biomimetic strategy to promote the regeneration of bone defects. Int J Nanomedicine. 2016; 11:2053–2067.17. Zhang J, Deng A, Yang Y, Gao L, Xu N, Liu X, Hu L, Chen J, Yang S. HPLC detection of loss rate and cell migration of HUVECs in a proanthocyanidin cross-linked recombinant human collagen-peptide (RHC)-chitosan scaffold. Mater Sci Eng C Mater Biol Appl. 2015; 56:555–563.
Article18. Fortunati D, Chau DY, Wang Z, Collighan RJ, Griffin M. Cross-linking of collagen I by tissue transglutaminase provides a promising biomaterial for promoting bone healing. Amino Acids. 2014; 46:1751–1761.
Article19. Castellan CS, Bedran-Russo AK, Antunes A, Pereira PN. Effect of dentin biomodification using naturally derived collagen cross-linkers: one-year bond strength study. Int J Dent. 2013; 2013:918010.
Article20. Ma L, Gao C, Mao Z, Zhou J, Shen J. Enhanced biological stability of collagen porous scaffolds by using amino acids as novel cross-linking bridges. Biomaterials. 2004; 25:2997–3004.
Article21. Bedran-Russo AK, Pauli GF, Chen SN, McAlpine J, Castellan CS, Phansalkar RS, Aguiar TR, Vidal CM, Napotilano JG, Nam JW, Leme AA. Dentin biomodification: strategies, renewable resources and clinical applications. Dent Mater. 2014; 30:62–76.
Article22. Bagchi D, Bagchi M, Stohs Sj, Ray SD, Sen CK, Preuss HG. Cellular protection with proanthocyanidins derived from grape seeds. Ann N Y Acad Sci. 2002; 957:260–270.
Article23. Ye X, Krohn RL, Liu W, Joshi SS, Kuszynski CA, McGinn TR, Bagchi M, Preuss HG, Stohs SJ, Bagchi D. The cytotoxic effects of a novel IH636 grape seed proanthocyanidin extract on cultured human cancer cells. Mol Cell Biochem. 1999; 196:99–108.
Article24. Kato A, Miyaji H, Ishizuka R, Tokunaga K, Inoue K, Kosen Y, Yokoyama H, Sugaya T, Tanaka S, Sakagami R, Kawanami M. Combination of root surface modification with BMP-2 and collagen hydrogel scaffold implantation for periodontal healing in beagle dogs. Open Dent J. 2015; 9:52–59.
Article25. Zhu W, Zhang Q, Zhang Y, Cen L, Wang J. PDL regeneration via cell homing in delayed replantation of avulsed teeth. J Transl Med. 2015; 13:357.
Article26. Han B, Jaurequi J, Tang BW, Nimni ME. Proanthocyanidin: a natural crosslinking reagent for stabilizing collagen matrices. J Biomed Mater Res A. 2003; 65:118–124.
Article27. Deters A, Dauer A, Schnetz E, Fartasch M, Hensel A. High molecular compounds (polysaccharides and proanthocyanidins) from Hamamelis virginiana bark: influence on human skin keratinocyte proliferation and differentiation and influence on irritated skin. Phytochemistry. 2001; 58:949–958.
Article28. Neuwirt H, Arias MC, Puhr M, Hobisch A, Culig Z. Oligomeric proanthocyanidin complexes (OPC) exert anti-proliferative and pro-apoptotic effects on prostate cancer cells. Prostate. 2008; 68:1647–1654.
Article29. Xie ZY, Wu BH, Yang ZG, Chen XF, Chen QS. Differentiation of human promyelocytic leukemia HL-60 cells induced by proanthocyanidin and its mechanism. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2013; 21:920–925.30. Madaghiele M, Calò E, Salvatore L, Bonfrate V, Pedone D, Frigione M, Sannino A. Assessment of collagen crosslinking and denaturation for the design of regenerative scaffolds. J Biomed Mater Res A. 2016; 104:186–194.
Article31. Faia-Torres AB, Guimond-Lischer S, Rottmar M, Charnley M, Goren T, Maniura-Weber K, Spencer ND, Reis RL, Textor M, Neves NM. Differential regulation of osteogenic differentiation of stem cells on surface roughness gradients. Biomaterials. 2014; 35:9023–9032.
Article32. Kwon YS, Lim ES, Kim HM, Hwang YC, Lee KW, Min KS. Genipin, a cross-linking agent, promotes odontogenic differentiation of human dental pulp cells. J Endod. 2015; 41:501–507.
Article33. Lim ES, Lim MJ, Min KS, Kwon YS, Hwang YC, Yu MK, Hong CU, Lee KW. Effects of epicatechin, a crosslinking agent, on human dental pulp cells cultured in collagen scaffolds. J Appl Oral Sci. 2016; 24:76–84.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Graded-Three-Dimensional Cell-Encapsulating Hydrogel as a Potential Biologic Scaffold for Disc Tissue Engineering
- Mechanically Reinforced Extracellular Matrix Scaffold for Application of Cartilage Tissue Engineering
- Simple and Novel Three Dimensional Neuronal Cell Culture Using a Micro Mesh Scaffold
- The Effect of the Mechanical Properties of the 3D Printed Gelatin/ Hyaluronic Acid Scaffolds on hMSCs Differentiation Towards Chondrogenesis
- Effects of Type I Collagen Concentration in Hydrogel on the Growth and Phenotypic Expression of Rat Chondrocytes