Allergy Asthma Immunol Res.
2017 Jan;9(1):52-60. 10.4168/aair.2017.9.1.52.
Asthma Severity and the Controller Prescription in Children at 12 Tertiary Hospitals
- Affiliations
-
- 1Department of Pediatrics, Seoul National University College of Medicine, Seoul, Korea.
- 2Department of Pediatrics, Soonchunhyang University Hospital, Seoul, Korea.
- 3Department of Pediatrics, Gangneung Asan Hospital, University of Ulsan College of Medicine, Gangneung, Korea.
- 4Department of Pediatrics, Gangnam CHA Hospital, Seoul, Korea.
- 5Department of Pediatrics, Sacred Heart Hospital, Hallym University College of Medicine, Anyang, Korea.
- 6Department of Pediatrics, Gwanghye General Hospital, Busan, Korea.
- 7Department of Pediatrics, Seoul Paik Hospital, Inje University College of Medicine, Seoul, Korea.
- 8Department of Pediatrics, Inje University Sanggye Paik Hospital, Seoul, Korea.
- 9Department of Pediatrics, Kangdong Sacred Heart Hospital, Hallym University College of Medicine, Seoul, Korea.
- 10Department of Pediatrics, Kangwon National University Hospital, Chuncheon, Korea.
- 11Department of Pediatrics, Uijeongbu St. Mary's Hospital, The Catholic University of Korea, College of Medicine, Uijeongbu, Korea. jintackk@catholic.ac.kr
- 12Department of Pediatrics, Inha University Hospital, Incheon, Korea. dhnlim@naver.com
- 13Environmental Health Center for Allergic Rhinitis, Ministry of Environment, Korea.
- KMID: 2355894
- DOI: http://doi.org/10.4168/aair.2017.9.1.52
Abstract
- PURPOSE
Guidelines need to be tailored to where they are applied. We aimed to describe the distinctive asthma severity profile and the pattern of controller prescription in Korean children.
METHODS
Twelve pediatric allergists from tertiary medical centers reviewed medical records of all asthmatic children who visited their clinics between September 1 and November 30 of 2013. Controller prescriptions were re-classified into 4 categories, then the prevalence of each asthma severity category and the controller prescription patterns according to asthma severity assessed by a Western (Global Initiative for Asthma, GINA) and an Asia-Pacific (Japanese Pediatric GuideLine, JPGL) guideline were evaluated.
RESULTS
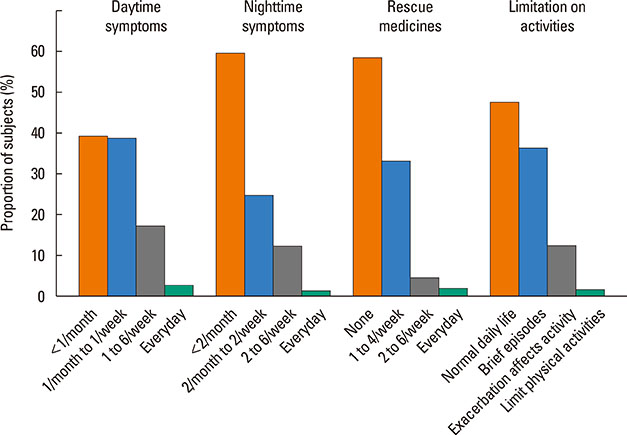
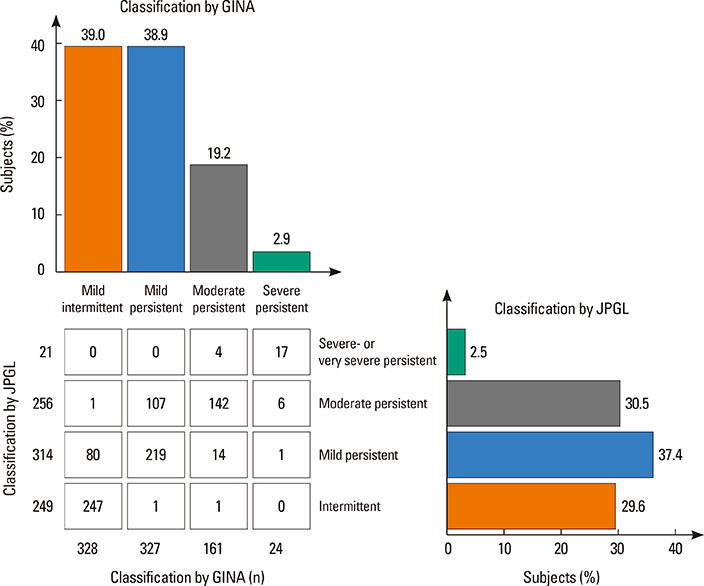
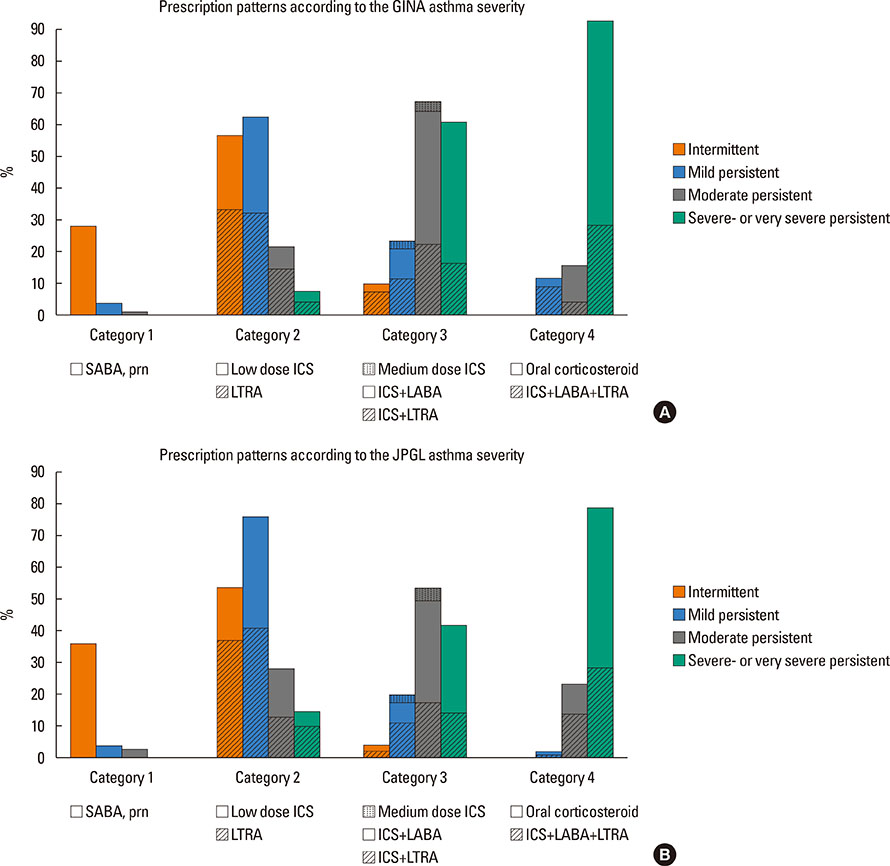
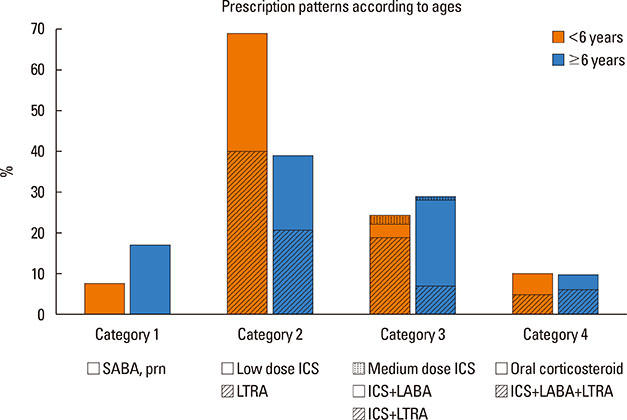
A total of 840 cases were reviewed. Both GINA and JPGL revealed that 328 (39.0%) and 249 (29.6%) subjects had intermittent asthma whereas 24 (2.9%) and 21 (2.5%) subjects had severe persistent asthma, respectively. Although higher category controllers tended to be prescribed to those who had more severe asthma, there was much overlap in categories of prescribed controllers between groups with regard to asthma severities. Leukotriene receptor antagonists (LTRA) was the most frequently prescribed as a single controller (40%) or as an add-on medication (19%) in the group of asthmatic children <6 years.
CONCLUSIONS
Korean children have distinctive patterns of asthma severity and management strategies with a lower prevalence of severe asthma and a preference toward LTRA rather than low dose inhaled corticosteroids (ICS) alone or add-on long-acting beta-agonist (LABA) in the group of <6 year-old asthmatics that has not been predicted in Western countries. Thus, strategies tailored to regional situations need to be developed and recommended.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
The Korean Version of the Test for Respiratory and Asthma Control in Kids (TRACK): Reliability and Validity
Yun Jung Choi, Gwang Cheon Jang, Hyeon-Jong Yang, Hyo-Bin Kim, Young Yoo, Meeyong Shin, So-Yeon Lee, Jakyoung Kim, Woo Kyung Kim, Dong In Suh, Young Yull Koh,
J Korean Med Sci. 2019;34(3):. doi: 10.3346/jkms.2019.34.e25.Studies and proposals of childhood asthma in Korea
Woo Kyung Kim
Allergy Asthma Respir Dis. 2018;6(Suppl 1):S52-S57. doi: 10.4168/aard.2018.6.S1.S52.
Reference
-
1. Bateman ED, Boushey HA, Bousquet J, Busse WW, Clark TJ, Pauwels RA, et al. Can guideline-defined asthma control be achieved? The Gaining Optimal Asthma ControL study. Am J Respir Crit Care Med. 2004; 170:836–844.2. National Asthma Education and Prevention Program (US). Expert panel report 2: guidelines for the diagnosis and management of asthma 1997. Bethesda (MD): National Institutes of Health, National Heart, Lung, and Blood Institute;1997.3. de Marco R, Marcon A, Jarvis D, Accordini S, Almar E, Bugiani M, et al. Prognostic factors of asthma severity: a 9-year international prospective cohort study. J Allergy Clin Immunol. 2006; 117:1249–1256.4. Myers TR. Guidelines for asthma management: a review and comparison of 5 current guidelines. Respir Care. 2008; 53:751–767.5. Lai CK, Beasley R, Crane J, Foliaki S, Shah J, Weiland S. International Study of Asthma and Allergies in Childhood Phase Three Study Group. Global variation in the prevalence and severity of asthma symptoms: phase three of the International Study of Asthma and Allergies in Childhood (ISAAC). Thorax. 2009; 64:476–483.6. Lai CK, De Guia TS, Kim YY, Kuo SH, Mukhopadhyay A, Soriano JB, et al. Asthma control in the Asia-Pacific region: the Asthma Insights and Reality in Asia-Pacific Study. J Allergy Clin Immunol. 2003; 111:263–268.7. Kondo N, Nishimuta T, Nishima S, Morikawa A, Aihara Y, Akasaka T, et al. Japanese pediatric guidelines for the treatment and management of bronchial asthma 2008. Pediatr Int. 2010; 52:319–326.8. Global Initiative for Asthma. GINA report, global strategy for asthma management and prevention: 2006 revision [Internet]. [place unknown]: Global Initiative for Asthma;2007. cited 2015 Dec 6. Available from: http://www.who.int/respiratory/asthma/GINA_WR_2006_copyright%5B1%5D.pdf.9. National Asthma Education and Prevention Program. Expert panel report 3 (EPR-3): guidelines for the diagnosis and management of asthma-summary report 2007. J Allergy Clin Immunol. 2007; 120:S94–S138.10. Jusuf L, Hsieh CT, Abad L, Chaiyote W, Chin WS, Choi YJ, et al. Primary care challenges in treating paediatric asthma in the Asia-Pacific region. Prim Care Respir J. 2013; 22:360–362.11. Chiu KC, Boonsawat W, Cho SH, Cho YJ, Hsu JY, Liam CK, et al. Patients' beliefs and behaviors related to treatment adherence in patients with asthma requiring maintenance treatment in Asia. J Asthma. 2014; 51:652–659.12. Guilbert TW, Morgan WJ, Zeiger RS, Bacharier LB, Boehmer SJ, Krawiec M, et al. Atopic characteristics of children with recurrent wheezing at high risk for the development of childhood asthma. J Allergy Clin Immunol. 2004; 114:1282–1287.13. Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005; 26:319–338.14. Global Initiative for Asthma. GINA report, global strategy for asthma management and prevention: 2014 [Internet]. Global Initiative for Asthma;2007. cited 2016 Jul 8. Available from: http://www.ginasthma.org/2014-GINA-Report%2C-Global-Strategy-for-Asthma-Management-and-Prevention.15. Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011; 184:602–615.16. Cho HJ, Jung YH, Yang SI, Lee E, Kim HY, Seo JH, et al. Reference values and determinants of fractional concentration of exhaled nitric oxide in healthy children. Allergy Asthma Immunol Res. 2014; 6:169–174.17. Song WJ, Kwon JW, Kim EJ, Lee SM, Kim SH, Lee SY, et al. Clinical application of exhaled nitric oxide measurements in a korean population. Allergy Asthma Immunol Res. 2015; 7:3–13.18. Taylor DR, Bateman ED, Boulet LP, Boushey HA, Busse WW, Casale TB, et al. A new perspective on concepts of asthma severity and control. Eur Respir J. 2008; 32:545–554.19. Ahn K, Kim J, Kwon HJ, Chae Y, Hahm MI, Lee KJ, et al. The prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in Korean children: nationwide cross-sectional survey using complex sampling design. J Korean Med Assoc. 2011; 54:769–778.20. Kim HY, Kwon EB, Baek JH, Shin YH, Yum HY, Jee HM, et al. Prevalence and comorbidity of allergic diseases in preschool children. Korean J Pediatr. 2013; 56:338–342.21. Kim YH, Urm SH, Kim WK. Prevalence of allergic diseases and risk factors in preschool children, 2009. Pediatr Allergy Respir Dis. 2011; 21:165–175.22. Cho SH, Kim YK, Chang YS, Kim SS, Min KU, Kim YY. Asthma insights and reality in Korea. Korean J Med. 2006; 70:69–77.23. Wong GW, Kwon N, Hong JG, Hsu JY, Gunasekera KD. Pediatric asthma control in Asia: phase 2 of the Asthma Insights and Reality in Asia-Pacific (AIRIAP 2) survey. Allergy. 2013; 68:524–530.24. Rabe KF, Adachi M, Lai CK, Soriano JB, Vermeire PA, Weiss KB, et al. Worldwide severity and control of asthma in children and adults: the global asthma insights and reality surveys. J Allergy Clin Immunol. 2004; 114:40–47.25. Lang A, Carlsen KH, Haaland G, Devulapalli CS, Munthe-Kaas M, Mowinckel P, et al. Severe asthma in childhood: assessed in 10 year olds in a birth cohort study. Allergy. 2008; 63:1054–1060.26. Sears MR. Epidemiology of asthma exacerbations. J Allergy Clin Immunol. 2008; 122:662–668.27. Johnston NW, Johnston SL, Duncan JM, Greene JM, Kebadze T, Keith PK, et al. The September epidemic of asthma exacerbations in children: a search for etiology. J Allergy Clin Immunol. 2005; 115:132–138.28. Kim E, Kim MJ, Lee JS, Yoon JS. Association between autumnal exacerbation and dermatophagoides pteronyssinus specific IgE in childhood asthma. Pediatr Allergy Respir Dis. 2007; 17:242–248.29. Graham LM. Classifying asthma. Chest. 2006; 130:13S–20S.30. Moon S, Shin J. Performance of universal health insurance: lessons from South Korea. World Health Popul. 2007; 9:95–113.31. Jones C, Santanello NC, Boccuzzi SJ, Wogen J, Strub P, Nelsen LM. Adherence to prescribed treatment for asthma: evidence from pharmacy benefits data. J Asthma. 2003; 40:93–101.32. Papi A, Corradi M, Pigeon-Francisco C, Baronio R, Siergiejko Z, Petruzzelli S, et al. Beclometasone-formoterol as maintenance and reliever treatment in patients with asthma: a double-blind, randomised controlled trial. Lancet Respir Med. 2013; 1:23–31.33. Bisgaard H, Le Roux P, Bjåmer D, Dymek A, Vermeulen JH, Hultquist C. Budesonide/formoterol maintenance plus reliever therapy: a new strategy in pediatric asthma. Chest. 2006; 130:1733–1743.34. Martinez FD, Chinchilli VM, Morgan WJ, Boehmer SJ, Lemanske RF Jr, Mauger DT, et al. Use of beclomethasone dipropionate as rescue treatment for children with mild persistent asthma (TREXA): a randomised, double-blind, placebo-controlled trial. Lancet. 2011; 377:650–657.35. Yawn BP, Brenneman SK, Allen-Ramey FC, Cabana MD, Markson LE. Assessment of asthma severity and asthma control in children. Pediatrics. 2006; 118:322–329.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Changes in the Indices of Bronchial Reversibility Assessed by the Office Spirometry and Their Relationship to Asthma Symptoms after Discontinuing Controller Medication in Children with Controlled Asthma: Pilot Study
- Analysis of Tertiary Hospital Utilization in Pediatric Orthopaedics: a Study Using Nationwide Sample Data from Korea
- Epidemiologic Study on Severity and Treatment of Childhood Asthma in Incheon, Korea
- Studies and proposals of childhood asthma in Korea
- Pharmacologic Treatment of Childhood Asthma