J Korean Med Sci.
2016 Dec;31(12):1879-1886. 10.3346/jkms.2016.31.12.1879.
Efficacy of Ginseng Supplements on Fatigue and Physical Performance: a Meta-analysis
- Affiliations
-
- 1Department of Cancer Control and Policy, Graduate School of Cancer Science and Policy, National Cancer Center, Goyang, Korea. msk@ncc.re.kr
- 2Molecular Epidemiology Branch, Research Institute, National Cancer Center, Goyang, Korea.
- 3Department of Family Medicine, Center for Cancer Prevention and Detection, National Cancer Center, Goyang, Korea.
- KMID: 2355614
- DOI: http://doi.org/10.3346/jkms.2016.31.12.1879
Abstract
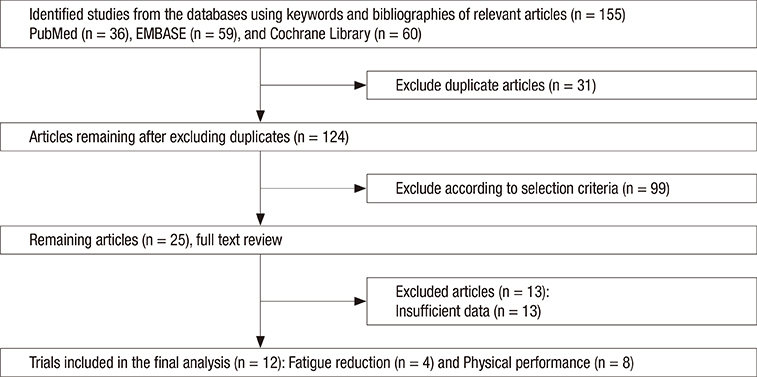
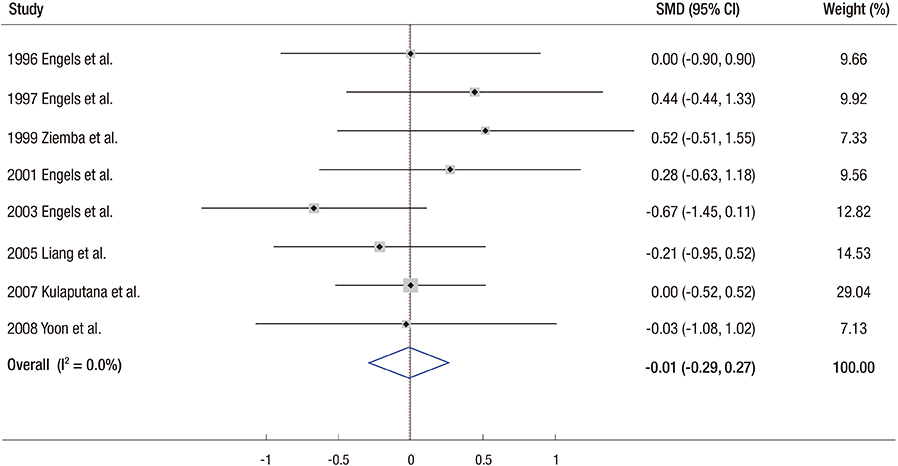
- We conducted a meta-analysis to investigate the efficacy of ginseng supplements on fatigue reduction and physical performance enhancement as reported by randomized controlled trials (RCTs). RCTs that investigated the efficacy of ginseng supplements on fatigue reduction and physical performance enhancement compared with placebos were included. The main outcome measures were fatigue reduction and physical performance enhancement. Out of 155 articles meeting initial criteria, 12 RCTs involving 630 participants (311 participants in the intervention group and 319 participants in the placebo group) were included in the final analysis. In the fixed-effect meta-analysis of four RCTs, there was a statistically significant efficacy of ginseng supplements on fatigue reduction (standardized mean difference, SMD = 0.34; 95% confidence interval [CI] = 0.16 to 0.52). However, ginseng supplements were not associated with physical performance enhancement in the fixed-effect meta-analysis of eight RCTs (SMD = −0.01; 95% CI = −0.29 to 0.27). We found that there was insufficient clinical evidence to support the use of ginseng supplements on reducing fatigue and enhancing physical performance because only few RCTs with a small sample size have been published so far. Further lager RCTs are required to confirm the efficacy of ginseng supplements on fatigue reduction.
Keyword
Figure
Cited by 1 articles
-
The Law for Health Functional Foods, It is Time to Repeal
Seung-Kwon Myung
J Korean Diabetes. 2017;18(4):205-213. doi: 10.4093/jkd.2017.18.4.205.
Reference
-
1. U.S. National Library of Medicine. MedlinePlus: fatigue [Internet]. accessed on 9 November 2015. Available at https://www.nlm.nih.gov/medlineplus/ency/article/003088.htm.2. Jones JF, Maloney EM, Boneva RS, Jones AB, Reeves WC. Complementary and alternative medical therapy utilization by people with chronic fatiguing illnesses in the United States. BMC Complement Altern Med. 2007; 7:12.3. EFSA Panel on Dietetic Products. Nutrition and Allergies (NDA). Scientific opinion on the substantiation of a health claim related to beta-alanine and increase in physical performance during short-duration, high-intensity exercise pursuant to Article 13(5) of Regulation (EC) No 1924/2006. EFSA J. 2014; 12:3755.4. Baeg IH, So SH. The world ginseng market and the ginseng (Korea). J Ginseng Res. 2013; 37:1–7.5. Sticher O. Biochemical, pharmaceutical, and medical perspectives of ginseng. In : Lawson LD, Bauer R, editors. Phytomedicines of Europe: Chemistry and Biological Activity. Washington, D.C.: American Chemical Society;1998. p. 221–240.6. Blumenthal M, Busse WR. The Complete German Commission E Monographs: Therapeutic Guide to Herbal Medicines. Austin, TX: American Botanical Council;1998.7. Vuksan V, Sievenpiper JL, Xu Z, Wong EY, Jenkins AL, Beljan-Zdravkovic U, Leiter LA, Josse RG, Stavro MP. Konjac-Mannan and American ginsing: emerging alternative therapies for type 2 diabetes mellitus. J Am Coll Nutr. 2001; 20:370S–80S.8. Allergy Research Group. Natural standard monographs: Panax ginseng. accessed on 10 November 2015. Available at http://www.allergyresearchgroup.com/monographs.9. Lee NH, Son CG. Systematic review of randomized controlled trials evaluating the efficacy and safety of ginseng. J Acupunct Meridian Stud. 2011; 4:85–97.10. Shim JY, Kim MH, Kim HD, Ahn JY, Yun YS, Song JY. Protective action of the immunomodulator ginsan against carbon tetrachloride-induced liver injury via control of oxidative stress and the inflammatory response. Toxicol Appl Pharmacol. 2010; 242:318–325.11. Suh SO, Kroh M, Kim NR, Joh YG, Cho MY. Effects of red ginseng upon postoperative immunity and survival in patients with stage III gastric cancer. Am J Chin Med. 2002; 30:483–494.12. Kim E, Cameron M, Lovera J, Schaben L, Bourdette D, Whitham R. American ginseng does not improve fatigue in multiple sclerosis: a single center randomized double-blind placebo-controlled crossover pilot study. Mult Scler. 2011; 17:1523–1526.13. Barton DL, Liu H, Dakhil SR, Linquist B, Sloan JA, Nichols CR, McGinn TW, Stella PJ, Seeger GR, Sood A, et al. Wisconsin Ginseng (Panax quinquefolius) to improve cancer-related fatigue: a randomized, double-blind trial, N07C2. J Natl Cancer Inst. 2013; 105:1230–1238.14. Etemadifar M, Sayahi F, Abtahi SH, Shemshaki H, Dorooshi GA, Goodarzi M, Akbari M, Fereidan-Esfahani M. Ginseng in the treatment of fatigue in multiple sclerosis: a randomized, placebo-controlled, double-blind pilot study. Int J Neurosci. 2013; 123:480–486.15. Kim HG, Cho JH, Yoo SR, Lee JS, Han JM, Lee NH, Ahn YC, Son CG. Antifatigue effects of Panax ginseng C.A. Meyer: a randomised, double-blind, placebo-controlled trial. PLoS One. 2013; 8:e61271.16. Engels H, Said JM, Wirth JC. Failure of chronic ginseng supplementation to affect work performance and energy metabolism in healthy adult females. Nutr Res. 1996; 16:1295–1305.17. Engels HJ, Wirth JC. No ergogenic effects of ginseng (Panax ginseng C.A. Meyer) during graded maximal aerobic exercise. J Am Diet Assoc. 1997; 97:1110–1115.18. Ziemba AW, Chmura J, Kaciuba-Uscilko H, Nazar K, Wisnik P, Gawronski W. Ginseng treatment improves psychomotor performance at rest and during graded exercise in young athletes. Int J Sport Nutr. 1999; 9:371–377.19. Engels HJ, Kolokouri I, Cieslak TJ 2nd, Wirth JC. Effects of ginseng supplementation on supramaximal exercise performance and short-term recovery. J Strength Cond Res. 2001; 15:290–295.20. Engels HJ, Fahlman MM, Wirth JC. Effects of ginseng on secretory IgA, performance, and recovery from interval exercise. Med Sci Sports Exerc. 2003; 35:690–696.21. Liang MT, Podolka TD, Chuang WJ. Panax notoginseng supplementation enhances physical performance during endurance exercise. J Strength Cond Res. 2005; 19:108–114.22. Kulaputana O, Thanakomsirichot S, Anomasiri W. Ginseng supplementation does not change lactate threshold and physical performances in physically active Thai men. J Med Assoc Thai. 2007; 90:1172–1179.23. Yoon SJ, Kim KH, Kim CJ, Park HC, Kang KH, Kim MJ, Kang SM, Kwak UH, Kim HJ. Effects of red ginseng supplementation on aerobic · anaerobic performance, central and peripheral fatigue. J Ginseng Res. 2008; 32:210–219.24. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996; 17:1–12.25. Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions: version 5.1.0. updated March 2011. accessed on 9 November 2015. Avaiable at http://handbook.cochrane.org/.26. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002; 21:1539–1558.27. Cohen J. A power primer. Psychol Bull. 1992; 112:155–159.28. Scholey AB, Kennedy DO. Acute, dose-dependent cognitive effects of Ginkgo biloba, Panax ginseng and their combination in healthy young volunteers: differential interactions with cognitive demand. Hum Psychopharmacol. 2002; 17:35–44.29. Reay JL, Kennedy DO, Scholey AB. Single doses of Panax ginseng (G115) reduce blood glucose levels and improve cognitive performance during sustained mental activity. J Psychopharmacol. 2005; 19:357–365.30. Reay JL, Kennedy DO, Scholey AB. Effects of Panax ginseng, consumed with and without glucose, on blood glucose levels and cognitive performance during sustained ‘mentally demanding’ tasks. J Psychopharmacol. 2006; 20:771–781.31. Itoh T, Zang YF, Murai S, Saito H. Effects of Panax ginseng root on the vertical and horizontal motor activities and on brain monoamine-related substances in mice. Planta Med. 1989; 55:429–433.32. Samira MM, Attia MA, Allam M, Elwan O. Effect of the standardized Ginseng Extract G115 on the metabolism and electrical activity of the rabbit’s brain. J Int Med Res. 1985; 13:342–348.33. Kaku T, Miyata T, Uruno T, Sako I, Kinoshita A. Chemico-pharmacological studies on saponins of Panax ginseng C. A. Meyer. II. Pharmacological part. Arzneimittelforschung. 1975; 25:539–547.34. Lawrence ME, Kirby DF. Nutrition and sports supplements: fact or fiction. J Clin Gastroenterol. 2002; 35:299–306.35. Maughan RJ, King DS, Lea T. Dietary supplements. J Sports Sci. 2004; 22:95–113.36. Kim DH, Moon YS, Jung JS, Min SK, Son BK, Suh HW, Song DK. Effects of ginseng saponin administered intraperitoneally on the hypothalamo-pituitary-adrenal axis in mice. Neurosci Lett. 2003; 343:62–66.37. Zhang HG, Li XH, Yang ZC. Effects of Panax notoginseng saponins on myocardial Gsalpha mRNA expression and ATPase activity after severe scald in rats. Burns. 2003; 29:541–546.38. Bae JW, Lee MH. Effect and putative mechanism of action of ginseng on the formation of glycated hemoglobin in vitro. J Ethnopharmacol. 2004; 91:137–140.39. Li Z, Chen X, Niwa Y, Sakamoto S, Nakaya Y. Involvement of Ca2+ -activated K+ channels in ginsenosides-induced aortic relaxation in rats. J Cardiovasc Pharmacol. 2001; 37:41–47.40. Hsu CC, Ho MC, Lin LC, Su B, Hsu MC. American ginseng supplementation attenuates creatine kinase level induced by submaximal exercise in human beings. World J Gastroenterol. 2005; 11:5327–5331.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Lipids in Ginseng (Panax ginseng) and Their Analysis
- Evidence Based Dietary Supplements for Fatigue and Sexual Function
- Effect of Ginseng on Blood Pressure: A Systematic Review and Meta-Analysis
- Efficacy of Vitamin C Supplements in Prevention of Cancer: A Meta-Analysis of Randomized Controlled Trials
- Effects of Vitamin and Antioxidant Supplements in Prevention of Bladder Cancer: a Meta-Analysis of Randomized Controlled Trials