Nat Prod Sci.
2016 Sep;22(3):209-215. 10.20307/nps.2016.22.3.209.
Induction of Apoptosis with Kigelia africana fruits in HCT116 Human Colon Cancer Cells via MAPKs Signaling Pathway
- Affiliations
-
- 1College of Natural Sciences, Duksung Women's University, Seoul 01369, Republic of Korea. hasook@duksung.ac.kr
- KMID: 2355147
- DOI: http://doi.org/10.20307/nps.2016.22.3.209
Abstract
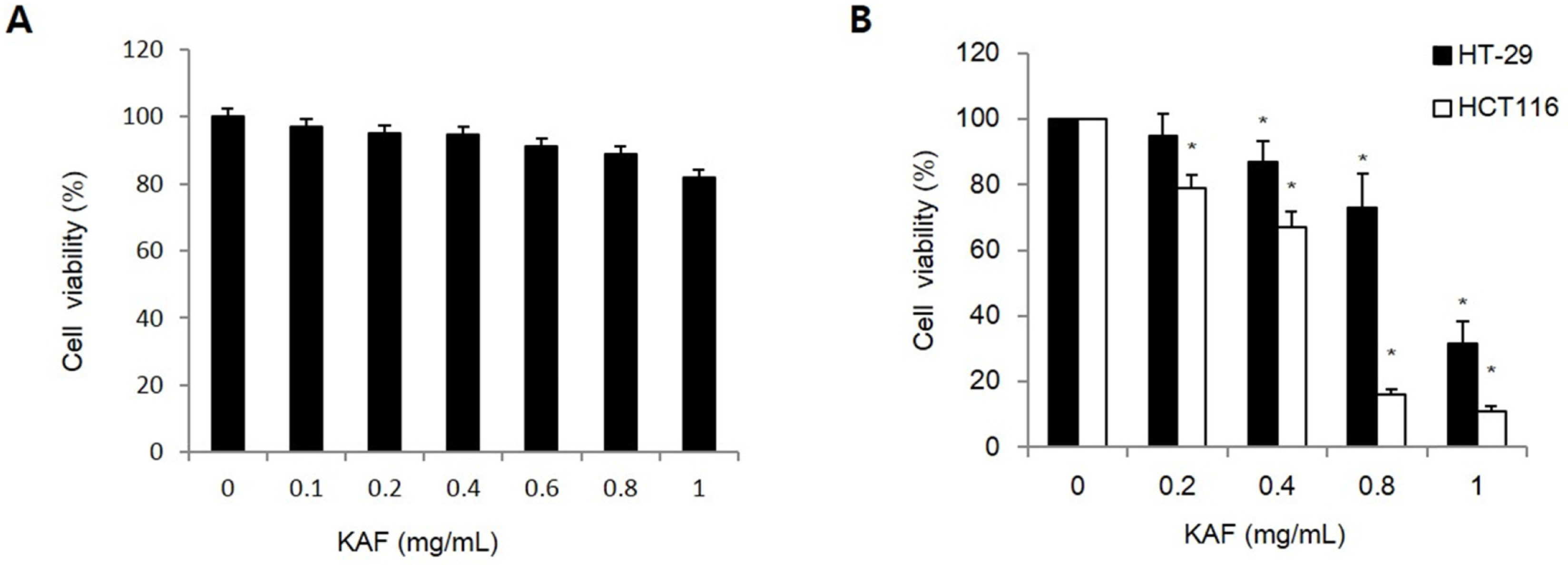
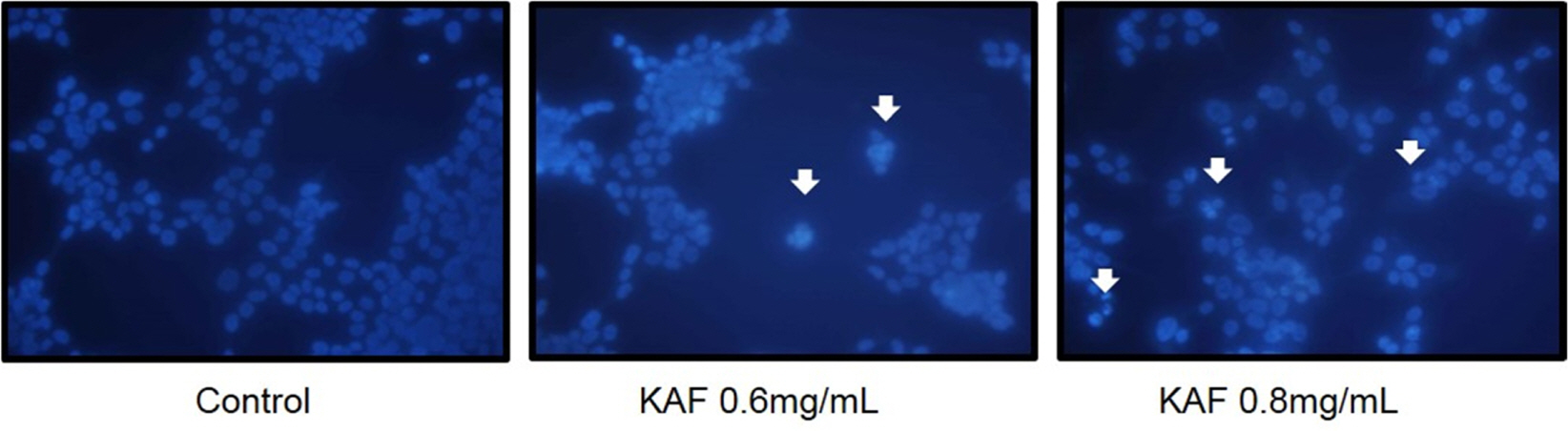
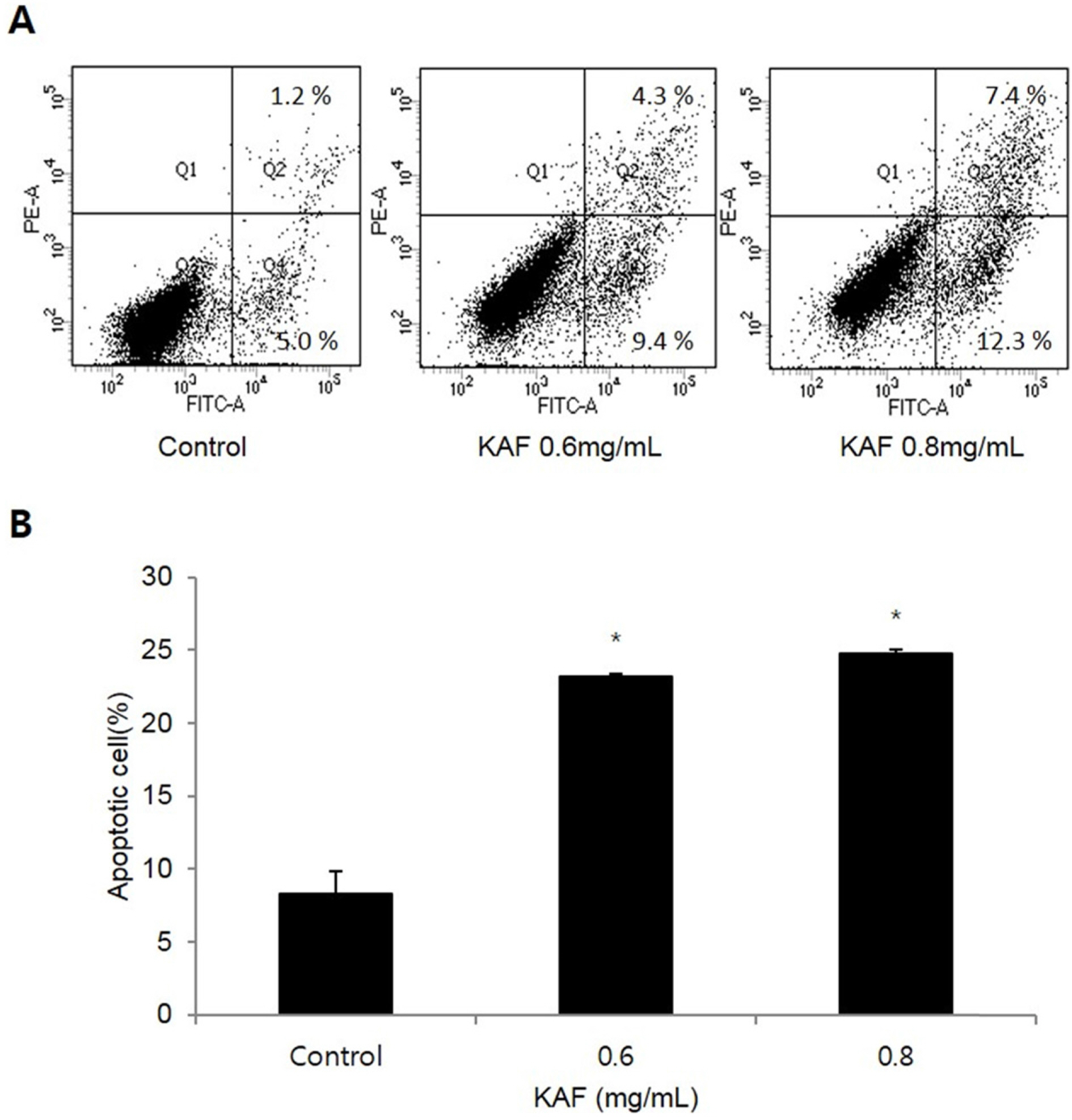
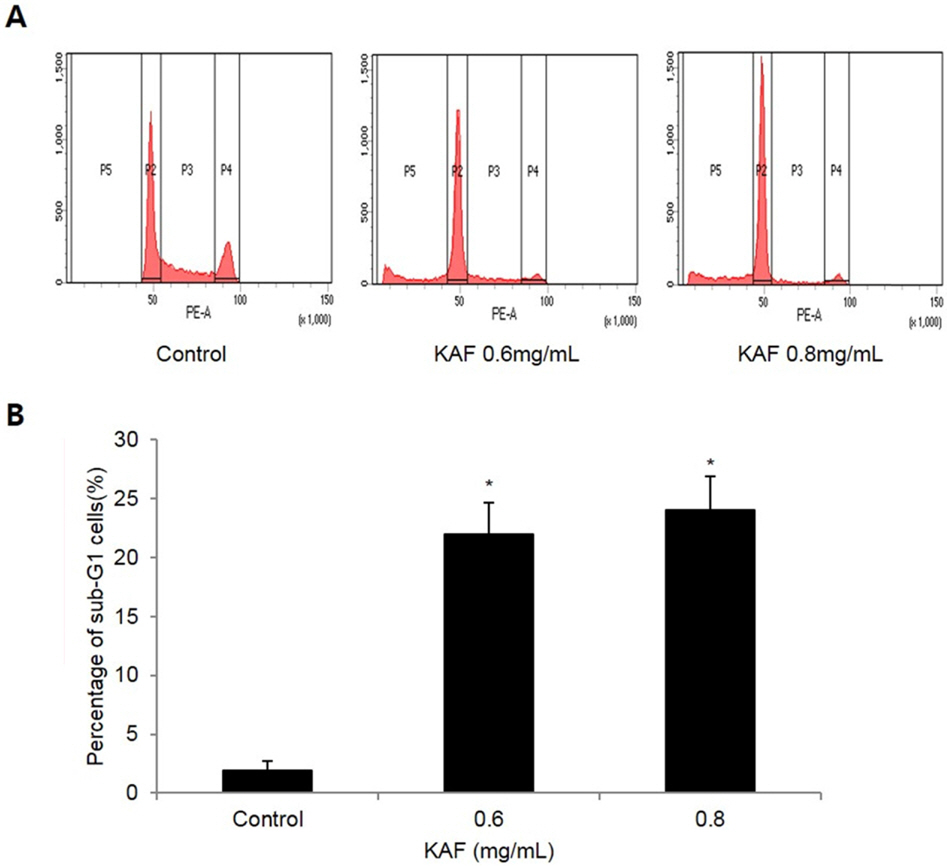
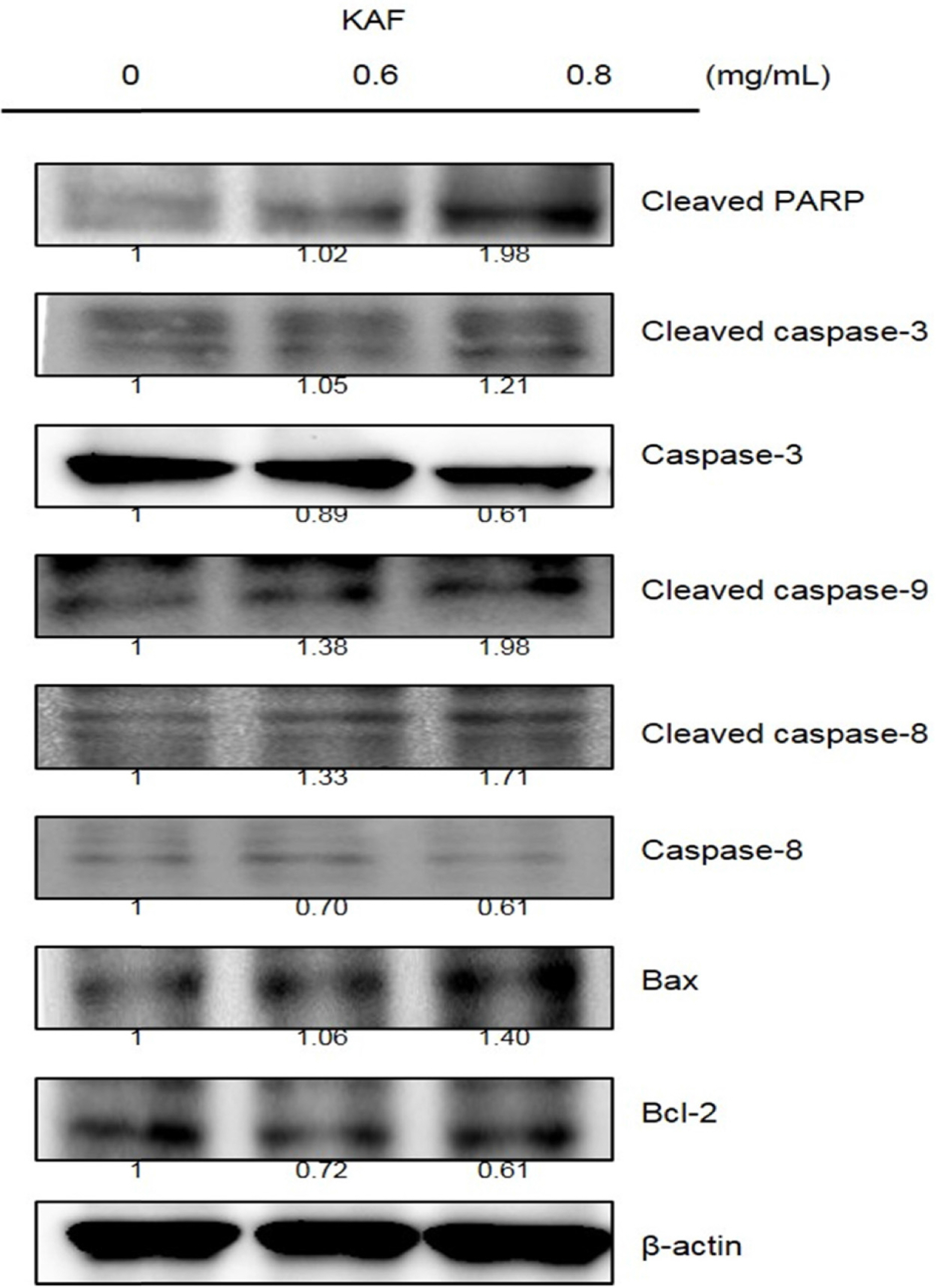
- Kigelia africana (Lam.) Benth. (Bignoniaceae) is a flowering plants in South, Central and West Africa and commonly known as the sausage tree (Eng.); worsboom (Afr.); umVunguta, umFongothi (Zulu); Modukguhlu (North Sotho); Muvevha (Venda). The dried, powdered fruits are used as dressing for wounds and ulcers, haemorrhoids, rheumatism, purgative, skin-firming, lactation in breast-feeding mothers. The aim of this study is to investigate the cytotoxic and apoptotic potentials of 70% ethanolic extracts of Kigelia africana fruits in HCT116 human colon cancer cells. Treatment of Kigelia africana fruits with various concentrations resulted in a sequence of characteristic of apoptosis, including loss of cell viability and morphological changes. Flow cytometry analysis showed Kigelia africana fruits increased the sub-G1 phase (apoptosis) population. Apoptosis confirmed by annexin V-fluorescein isothiocyanate and propidium iodide double staining in HCT116 human colon cancer cell lines. Moreover, analysis of the mechanism indicated that Kigelia africana fruits showed an increased Bax and Bcl-2 expressions in a dose-dependent manner, resulting in activation of hallmarks of apoptotic events, caspase-3, caspase-9 and cleaved poly-ADP-ribose polymerase. This is the first report to demonstrate the cytotoxicity of Kigelia africana fruits on HCT116 human colon cancer cells.
MeSH Terms
Figure
Reference
-
(1). Jemal A., Bray F., Center M. M., Ferlay J., Ward E., Forman D. CA. Cancer J. Clin. 2011; 61:69–90.(2). Line-Edwige M., Raymond F. G., Francois E., François E., Edouard N. E. Afr. J.Tradit. Complement. Altern. Med. 2009; 6:112–117.(3). Sun Q., Chen T., Wang X., Wei X. J.Cell Physiol. 2010; 222:421–432.
Article(4). Thornberry N. A., Rano T. A., Peterson E. P., Rasper D. M., Timkey T., Garcia-Calvo M., Houtzager V. M., Nordstrom P. A., Roy S., Vaillancourt J. P., Chapman K. T., Nicholson D. W. J.Biol. Chem. 1997; 272:17907–17911.(5). Wang X.Genes Dev. 2001; 15:2922–2933.(6). Reuter S., Eifes S., Dicato M., Aggarwal B. B., Diederich M.Biochem. Pharmacol. 2008; 76:1340–1351.(7). Balaban R. S., Nemoto S., Finket T.Cell. 2005; 120:483–495.(8). Madesh M., Antonsson B., Srinivasula S. M., Alnemri E. S., Hajnóczky G. J.Biol. Chem. 2002; 277:5651–5659.(9). Fernandes-Alnemri T., Litwack G., Alnemri E. S. J.Biol. Chem. 1994; 269:30761–30764.(10). Saini S., Kaur H., Verma B., Ripudaman Singh S. K.Nat. Prod. Rad. 2009; 8:190–197.(11). Higgins C. A., Bell T., Delbederi Z., Feutren-Burton S., McClean B., O'Dowd C., Watters W., Armstrong P., Waugh D., van den Berg H.Planta Med. 2010; 76:1840–1846.(12). Agyare C., Dwobeng A. S., Agyepong N., Boakye Y. D., Mensah K. B., Ayande P. G., Adarkwa-Yiadom M.See comment in PubMed Commons below Adv. Pharmacol. Sci. 2013; Article ID 692613. 10.(13). Picerno P., Autore G., Marzocco S., Meloni M., Sanogo R., Aquino R. P. J.Nat. Prod. 2005; 68:1610–1614.(14). Carmichael J., DeGraff W. G., Gazdar A. F., Minna J. D., Mitchell J. B.Cancer Res. 1987; 47:936–942.(15). Lizard G., Fournel S., Genestier L., Dhedin N., Chaput C., Flacher M., Mutin M., Panaye G., Revillard J. P.Cytometry. 1995; 21:275–283.(16). Nicoletti I., Migliorati G., Paqliacci M. C., Grignani F., Riccardi C. J.Immunol. Methods. 1991; 139:271–279.(17). Ryu M. J., Kim A. D., Kang K. A., Chung H. S., Kim H. S., Suh I. S., Chang W. Y., Hyun J. W.In Vitro Cell. Dev. Biol. Anim. 2013; 49:74–81.(18). Strickland L., Letson G. D., Muro-Cacho C. A.Cancer Control. 2001; 8:252–261.(19). Ryu M. J., Chung H. S.In Vitro Cell. Dev. Biol. Anim. 2015; 51:92–101.(20). Bold R. J., Termuhlen P. M., McConkey D. J.Surg. Oncol. 1997; 6:133–142.(21). Xu Y., Ge R., Du J., Xin H., Yi T., Sheng J., Wang Y., Ling C.Cancer Lett. 2009; 284:229–237.(22). Novák B., Tyson J. J.Biochem. Soc. Trans. 2003; 31:1526–1529.(23). Han C. R., Jun D. Y., Woo H. J., Jeong S. Y., Woo M. H., Kim Y. H.Bioorg. Med. Chem. Lett. 2012; 22:945–953.(24). Cory S., Adams J. M.Nat. Rev. Cancer. 2002; 2:647–656.(25). Nagappan A., Park K. I., Park H. S., Kim J. A., Hong G. E., Kang S. R., Lee D. H., Kim E. H., Lee W. S., Won C. K., Kim G. S.Food Chem. 2012; 135:1920–1928.(26). Kim K. N., Ham Y. M., Moon J. Y., Kim M. J., Jung Y. H., Jeon Y. J., Lee N. H., Kang N., Yang H. M., Kim D., Hyun C. G.Food Chem. 2012; 135:2112–2117.(27). Oliver F. J., de la Rubia G., Rolli V., Ruiz-Ruiz M. C., de Murcia G., Murcia J. M. J.Biol. Chem. 1998; 273:33533–33539.(28). Cuadrado A., Nebreda A. R.Biochem. J. 2010; 429:403–417.(29). Ahmed-Choudhury J., Williams K. T., Young L. S., Adams D. H., Afford S. C.Cell Signal. 2006; 18:456–468.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Induction of Apoptosis with Moringa oleifera Fruits in HCT116 Human Colon Cancer Cells Via Intrinsic Pathway
- Anti-Proliferative Activity of Nodosin, a Diterpenoid from Isodon serra, via Regulation of Wnt/β-Catenin Signaling Pathways in Human Colon Cancer Cells
- Vinpocetine inhibits the proliferation and induces apoptosis in human colon cancer cells
- Cucurbitacin E’s Anti-Cancer Effects on HCT116 Human Colon Cancer Cells by Controlling Expression and Phosphorylation Levels of Caspase-9, eIF-2α, and ATF-4
- The Changes of Expression of Survivin by Butyrate in HCT116 Colon Cancer Cells