J Clin Neurol.
2016 Apr;12(2):201-208. 10.3988/jcn.2016.12.2.201.
Regional MRI Diffusion, White-Matter Hyperintensities, and Cognitive Function in Alzheimer's Disease and Vascular Dementia
- Affiliations
-
- 1Neurology Unit, Campus Bio-Medico University, Rome, Italy. c.altamura@unicampus.it
- 2Radiology Unit, Campus Bio-Medico University, Rome, Italy.
- 3Clinical Medicine Department, Public Health, Life and Environment Scinces, University of L'Aquila, Italy.
- 4IRCCS S. Raffaele, Rome - S.Raffaele Clinic, Cassino (FR), Italy.
- 5Neurology Clinic, Università Politecnica delle Marche, Ancona, Italy.
- 6Neurology Clinic, Università Cattolica del Sacro Cuore, Rome, Italy.
- 7Associazione Fatebenefratelli per la ricerca-AFAR, Rome, Italy.
- 8Internal and Subintensive Medicine, Ospedali Riuniti Ancona, Italy.
- KMID: 2354141
- DOI: http://doi.org/10.3988/jcn.2016.12.2.201
Abstract
- BACKGROUND AND PURPOSE
An increase in brain water diffusivity as measured using magnetic resonance imaging (MRI) has been recently reported in normal-appearing white matter (NAWM) in patients affected by cognitive impairment. However, it remains to be clarified if this reflects an overt neuronal tissue disruption that leads to degenerative or microvascular lesions. This question was addressed by comparing the regional MRI apparent diffusion coefficients (ADCs) of NAWM in patients affected by Alzheimer's disease (AD) or vascular dementia (VaD). The relationships of ADCs with the white-matter hyperintensity (WMH) burden, carotid atherosclerosis, and cognitive performance were also investigated.
METHODS
Forty-nine AD and 31 VaD patients underwent brain MRI to assess the WMH volume and regional NAWM ADCs, neuropsychological evaluations, and carotid ultrasound to assess the plaque severity and intima-media thickness (IMT).
RESULTS
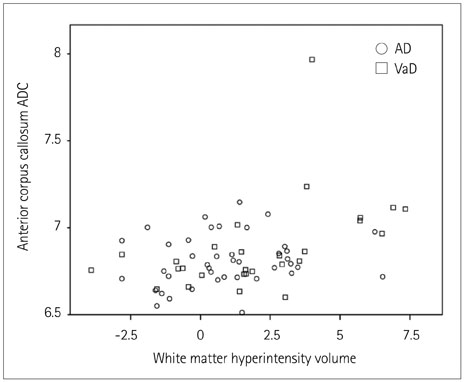
Regional ADCs in NAWM did not differ between VaD and AD patients, while the WMH volume was greater in VaD than in AD patients. The ADC in the anterior corpus callosum was related to the WMH volume, while a greater carotid IMT was positively correlated with the temporal ADC and WMH volume. The memory performance was worse in patients with higher temporal ADCs. Constructional praxis scores were related to ADCs in the frontal, and occipital lobes, in the anterior and posterior corpus callosum as well as to the WMH volume. Abstract reasoning was related to frontal, parietal, and temporal ADCs.
CONCLUSIONS
Our data show that higher regional ADCs in NAWM are associated with microcirculatory impairment, as depicted by the WMH volume. Moreover, regional ADCs in NAWM are differently associated with the neuropsychological performances in memory, constructional praxia, and abstract reasoning domains.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Medial-Vowel Writing Difficulty in Korean Syllabic Writing: A Characteristic Sign of Alzheimer's Disease
Ji Hye Yoon, Yong Jeong, Duk L. Na
J Clin Neurol. 2018;14(2):179-185. doi: 10.3988/jcn.2018.14.2.179.White-Matter Hyperintensities and Lacunar Infarcts Are Associated with an Increased Risk of Alzheimer's Disease in the Elderly in China
Shuai Ye, Shuyang Dong, Jun Tan, Le Chen, Hai Yang, Yang Chen, Zeyan Peng, Yingchao Huo, Juan Liu, Mingshan Tang, Yafei Li, Huadong Zhou, Yong Tao
J Clin Neurol. 2019;15(1):46-53. doi: 10.3988/jcn.2019.15.1.46.
Reference
-
1. Viticchi G, Falsetti L, Vernieri F, Altamura C, Bartolini M, Luzzi S, et al. Vascular predictors of cognitive decline in patients with mild cognitive impairment. Neurobiol Aging. 2012; 33:1127.e1–1127.e9.
Article2. Iadecola C. The overlap between neurodegenerative and vascular factors in the pathogenesis of dementia. Acta Neuropathol. 2010; 120:287–296.
Article3. van Oijen M, de Jong FJ, Witteman JC, Hofman A, Koudstaal PJ, Breteler MM. Atherosclerosis and risk for dementia. Ann Neurol. 2007; 61:403–410.
Article4. Altamura C, Squitti R, Pasqualetti P, Tibuzzi F, Silvestrini M, Ventriglia MC, et al. What is the relationship among atherosclerosis markers, apolipoprotein E polymorphism and dementia? Eur J Neurol. 2007; 14:679–682.
Article5. de la Torre JC. The vascular hypothesis of Alzheimer's disease: bench to bedside and beyond. Neurodegener Dis. 2010; 7:116–121.
Article6. Buratti L, Balestrini S, Altamura C, Viticchi G, Falsetti L, Luzzi S, et al. Markers for the risk of progression from mild cognitive impairment to Alzheimer's disease. J Alzheimers Dis. 2015; 45:883–890.
Article7. de la Torre JC. Carotid artery ultrasound and echocardiography testing to lower the prevalence of Alzheimer's disease. J Stroke Cerebrovasc Dis. 2009; 18:319–328.
Article8. Marshall RS, Lazar RM. Pumps, aqueducts, and drought management: vascular physiology in vascular cognitive impairment. Stroke. 2011; 42:221–226.9. O'Sullivan M, Morris RG, Huckstep B, Jones DK, Williams SC, Markus HS. Diffusion tensor MRI correlates with executive dysfunction in patients with ischaemic leukoaraiosis. J Neurol Neurosurg Psychiatry. 2004; 75:441–447.10. Shenkin SD, Bastin ME, Macgillivray TJ, Deary IJ, Starr JM, Rivers CS, et al. Cognitive correlates of cerebral white matter lesions and water diffusion tensor parameters in community-dwelling older people. Cerebrovasc Dis. 2005; 20:310–318.
Article11. Della Nave R, Foresti S, Pratesi A, Ginestroni A, Inzitari M, Salvadori E, et al. Whole-brain histogram and voxel-based analyses of diffusion tensor imaging in patients with leukoaraiosis: correlation with motor and cognitive impairment. AJNR Am J Neuroradiol. 2007; 28:1313–1319.
Article12. Nitkunan A, Barrick TR, Charlton RA, Clark CA, Markus HS. Multimodal MRI in cerebral small vessel disease: its relationship with cognition and sensitivity to change over time. Stroke. 2008; 39:1999–2005.13. Viana-Baptista M, Bugalho P, Jordão C, Ferreira N, Ferreira A, Forjaz Secca M, et al. Cognitive function correlates with frontal white matter apparent diffusion coefficients in patients with leukoaraiosis. J Neurol. 2008; 255:360–366.
Article14. Wang L, Goldstein FC, Levey AI, Lah JJ, Meltzer CC, Holder CA, et al. White matter hyperintensities and changes in white matter integrity in patients with Alzheimer's disease. Neuroradiology. 2011; 53:373–381.
Article15. Lee DY, Fletcher E, Martinez O, Ortega M, Zozulya N, Kim J, et al. Regional pattern of white matter microstructural changes in normal aging, MCI, and AD. Neurology. 2009; 73:1722–1728.
Article16. Gouw AA, Seewann A, Vrenken H, van der, Rozemuller JM, Barkhof F, et al. Heterogeneity of white matter hyperintensities in Alzheimer's disease: post-mortem quantitative MRI and neuropathology. Brain. 2008; 131(Pt 12):3286–3298.
Article17. Alves GS, O'Dwyer L, Jurcoane A, Oertel-Knöchel V, Knöchel C, Prvulovic D, et al. Different patterns of white matter degeneration using multiple diffusion indices and volumetric data in mild cognitive impairment and Alzheimer patients. PLoS One. 2012; 7:e52859.
Article18. Stahl R, Dietrich O, Teipel SJ, Hampel H, Reiser MF, Schoenberg SO. White matter damage in Alzheimer disease and mild cognitive impairment: assessment with diffusion-tensor MR imaging and parallel imaging techniques. Radiology. 2007; 243:483–492.
Article19. Kantarci K, Jack CR Jr, Xu YC, Campeau NG, O'Brien PC, Smith GE, et al. Mild cognitive impairment and Alzheimer disease: regional diffusivity of water. Radiology. 2001; 219:101–107.
Article20. Agosta F, Pievani M, Sala S, Geroldi C, Galluzzi S, Frisoni GB, et al. White matter damage in Alzheimer disease and its relationship to gray matter atrophy. Radiology. 2011; 258:853–863.
Article21. Kantarci K, Petersen RC, Boeve BF, Knopman DS, Weigand SD, O'Brien PC, et al. DWI predicts future progression to Alzheimer disease in amnestic mild cognitive impairment. Neurology. 2005; 64:902–904.
Article22. Staekenborg SS, Koedam EL, Henneman WJ, Stokman P, Barkhof F, Scheltens P, et al. Progression of mild cognitive impairment to dementia: contribution of cerebrovascular disease compared with medial temporal lobe atrophy. Stroke. 2009; 40:1269–1274.
Article23. Schmidt R, Ropele S, Ferro J, Madureira S, Verdelho A, Petrovic K, et al. Diffusion-weighted imaging and cognition in the leukoariosis and disability in the elderly study. Stroke. 2010; 41:e402–e408.
Article24. Maillard P, Carmichael O, Harvey D, Fletcher E, Reed B, Mungas D, et al. FLAIR and diffusion MRI signals are independent predictors of white matter hyperintensities. AJNR Am J Neuroradiol. 2013; 34:54–61.
Article25. de Groot M, Verhaaren BF, de Boer R, Klein S, Hofman A, van der Lugt A, et al. Changes in normal-appearing white matter precede development of white matter lesions. Stroke. 2013; 44:1037–1042.
Article26. Conklin J, Fierstra J, Crawley AP, Han JS, Poublanc J, Silver FL, et al. Mapping white matter diffusion and cerebrovascular reactivity in carotid occlusive disease. Neurology. 2011; 77:431–438.
Article27. Rentsch-Granges V, Assal F, Pereira VM, Alimenti A, Mosimann P, de Ribaupierre A, et al. ADC mapping of chronic cerebral hypoperfusion induced by carotid artery stenosis. J Neuroradiol. 2011; 38:232–237.
Article28. Soinne L, Helenius J, Saimanen E, Salonen O, Lindsberg PJ, Kaste M, et al. Brain diffusion changes in carotid occlusive disease treated with endarterectomy. Neurology. 2003; 61:1061–1065.
Article29. Sahin N, Solak A, Genc B, Akpinar MB, Kulu U, Cengiz H. Brain diffusion changes in unilateral carotid artery stenosis with non-shunt endarterectomy: correlation with white matter lesions. Clin Neurol Neurosurg. 2015; 133:24–29.
Article30. Dubois B, Feldman HH, Jacova C, Dekosky ST, Barberger-Gateau P, Cummings J, et al. Research criteria for the diagnosis of Alzheimer's disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007; 6:734–746.
Article31. Román GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology. 1993; 43:250–260.
Article32. van Straaten EC, Scheltens P, Knol DL, van Buchem MA, van Dijk EJ, Hofman PA, et al. Operational definitions for the NINDS-AIREN criteria for vascular dementia: an interobserver study. Stroke. 2003; 34:1907–1912.
Article33. Grigoletto F, Zappalà G, Anderson DW, Lebowitz BD. Norms for the Mini-Mental State Examination in a healthy population. Neurology. 1999; 53:315–320.
Article34. Miceli G, Laudanna A, Burani C, Capasso R. Batteria per l'Analisi dei Deficit Afasici (B.A.D.A.). Roma: CEPSAG;Università Cattolica del Sacro Cuore;1994.35. Spinnler H, Tognoni G. Standardizzazione e taratura italiana dei test neuropsicologici. Ital J Neurol Sci. 1987; 78-80:97–99.36. Lezak MD, Howieson DB, Loring DW. Neuropsychological assessment. 4th ed. New York: Oxford University Press;2004.37. Rosset A, Spadola L, Ratib O. OsiriX: an open-source software for navigating in multidimensional DICOM images. J Digit Imaging. 2004; 17:205–216.
Article38. Stejskal E, Tanner J. Spin diffusion measurements: spin echoes in the presence of a time-dependent field gradient. J Chem Phys. 1965; 42:288–292.
Article39. Touboul PJ, Hennerici MG, Meairs S, Adams H, Amarenco P, Bornstein N, et al. Mannheim carotid intima-media thickness consensus (2004-2006). An update on behalf of the Advisory Board of the 3rd and 4th Watching the Risk Symposium, 13th and 15th European Stroke Conferences, Mannheim, Germany, 2004, and Brussels, Belgium, 2006. Cerebrovasc Dis. 2007; 23:75–80.
Article40. Kantarci K, Senjem ML, Avula R, Zhang B, Samikoglu AR, Weigand SD, et al. Diffusion tensor imaging and cognitive function in older adults with no dementia. Neurology. 2011; 77:26–34.
Article41. Back SA, Kroenke CD, Sherman LS, Lawrence G, Gong X, Taber EN, et al. White matter lesions defined by diffusion tensor imaging in older adults. Ann Neurol. 2011; 70:465–476.
Article42. Pievani M, Agosta F, Pagani E, Canu E, Sala S, Absinta M, et al. Assessment of white matter tract damage in mild cognitive impairment and Alzheimer's disease. Hum Brain Mapp. 2010; 31:1862–1875.
Article43. Lee DY, Fletcher E, Martinez O, Zozulya N, Kim J, Tran J, et al. Vascular and degenerative processes differentially affect regional interhemispheric connections in normal aging, mild cognitive impairment, and Alzheimer disease. Stroke. 2010; 41:1791–1797.
Article44. Gootjes L, Teipel SJ, Zebuhr Y, Schwarz R, Leinsinger G, Scheltens P, et al. Regional distribution of white matter hyperintensities in vascular dementia, Alzheimer's disease and healthy aging. Dement Geriatr Cogn Disord. 2004; 18:180–188.
Article45. Kim HJ, Im K, Kwon H, Lee JM, Kim C, Kim YJ, et al. Clinical effect of white matter network disruption related to amyloid and small vessel disease. Neurology. 2015; 85:63–70.
Article46. Acosta-Cabronero J, Alley S, Williams GB, Pengas G, Nestor PJ. Diffusion tensor metrics as biomarkers in Alzheimer's disease. PLoS One. 2012; 7:e49072.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Association between Cognitive Function, Behavioral and Psychological Symptoms of Dementia and White Matter Hyperintensities in Patients with Alzheimer's Disease and Mild Cognitive Impairment
- The Association of Cognitive Dysfunction with White Matter Hyperintensity in Alzheimer's Disease and Mild Cognitive Impairment
- Micro-vascular Diseases of White Matter
- Effect of White Matter Hyperintensities on Daily Function via Depressive Symptoms: A Longitudinal Study in Patients With Dementia Including Alzheimer’s Disease and Subcortical Ischemic Vascular Dementia
- Impact of Hypertension on Cognitive Decline and Dementia