J Clin Neurol.
2016 Jul;12(3):323-331. 10.3988/jcn.2016.12.3.323.
Asymmetric Gray Matter Volume Changes Associated with Epilepsy Duration and Seizure Frequency in Temporal-Lobe-Epilepsy Patients with Favorable Surgical Outcome
- Affiliations
-
- 1Department of Neurology, Neuroscience Center, Samsung Medical Center and Samsung Advanced Institute for Health Sciences & Technology (SAIHST), Sungkyunkwan University School of Medicine, Seoul, Korea. sbhong@skku.edu
- 2Samsung Biomedical Research Institute (SBRI), Seoul, Korea.
- 3Department of Neurology, Seoul National University Boramae Hospital, Seoul, Korea.
- 4Department of Radiology, Samsung Medical Center, Sungkyunwan University School of Medicine, Seoul, Korea.
- KMID: 2354120
- DOI: http://doi.org/10.3988/jcn.2016.12.3.323
Abstract
- BACKGROUND AND PURPOSE
This study aimed to estimate the changes in gray matter volume (GMV) and their hemispheric difference in patients with mesial temporal lobe epilepsy (MTLE) using a voxel-based morphometry (VBM) methodology, and to determine whether GMV changes are correlated with clinical features.
METHODS
VBM analysis of brain MRI using statistical parametric mapping 8 (SPM8) was performed for 30 left MTLE (LMTLE) and 30 right MTLE (RMTLE) patients and 30 age- and sex-matched healthy controls. We also analyzed the correlations between GMV changes and clinical features of MTLE patients.
RESULTS
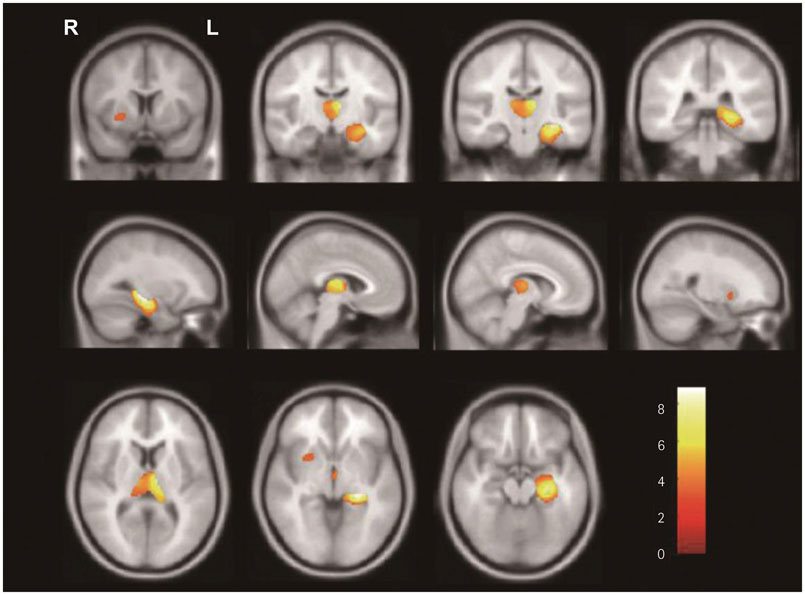
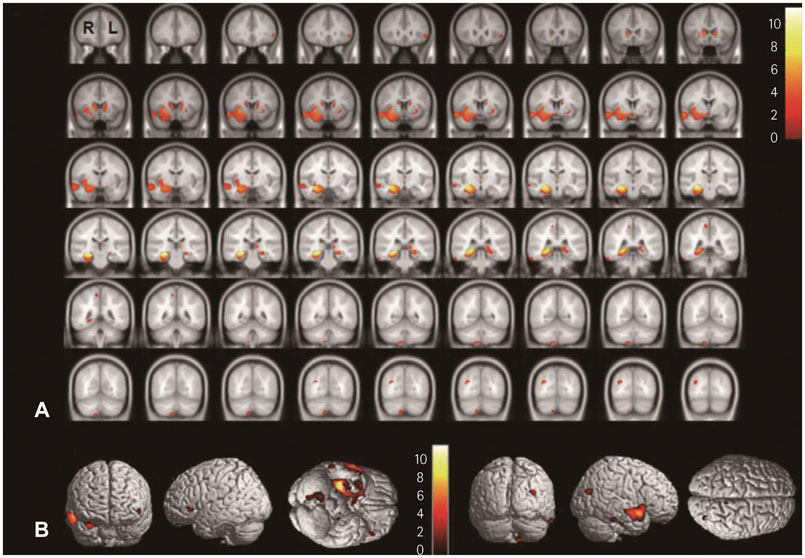
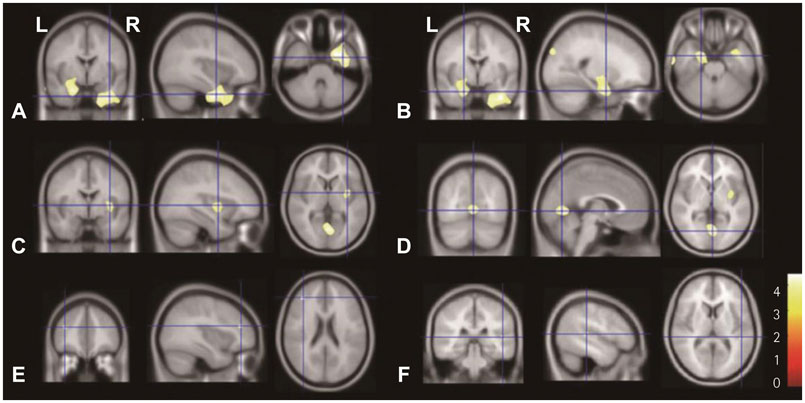
In SPM8-based analyses, MTLE patients showed significant GMV reductions in the hippocampus ipsilateral to the epileptic focus, bilateral thalamus, and contralateral putamen in LMTLE patients. The GMV reductions were more extensive in the ipsilateral hippocampus, thalamus, caudate, putamen, uncus, insula, inferior temporal gyrus, middle occipital gyrus, cerebellum, and paracentral lobule in RMTLE patients. These patients also exhibited notable reductions of GMV in the contralateral hippocampus, thalamus, caudate, putamen, and inferior frontal gyrus. We observed that GMV reduction was positively correlated with several clinical features (epilepsy duration and seizure frequency in RMTLE, and history of febrile seizure in LMTLE) and negatively correlated with seizure onset age in both the RMTLE and LMTLE groups.
CONCLUSIONS
Our study revealed GMV decreases in the hippocampus and extrahippocampal regions. Furthermore, the GMV reduction was more extensive in the RMTLE group than in the LMTLE group, since it included the contralateral hemisphere in the former. This difference in the GMV reduction patterns between LMTLE and RMTLE may be related to a longer epilepsy duration and higher seizure frequency in the latter.
Keyword
MeSH Terms
Figure
Reference
-
1. Bae EK, Jung KH, Chu K, Lee ST, Kim JH, Park KI, et al. Neuropathologic and clinical features of human medial temporal lobe epilepsy. J Clin Neurol. 2010; 6:73–80.
Article2. Regesta G, Tanganelli P. Clinical aspects and biological bases of drug-resistant epilepsies. Epilepsy Res. 1999; 34:109–122.
Article3. Keller SS, Roberts N. Voxel-based morphometry of temporal lobe epilepsy: an introduction and review of the literature. Epilepsia. 2008; 49:741–757.
Article4. Bernasconi N, Bernasconi A, Andermann F, Dubeau F, Feindel W, Reutens DC. Entorhinal cortex in temporal lobe epilepsy: a quantitative MRI study. Neurology. 1999; 52:1870–1876.5. Bernasconi N, Duchesne S, Janke A, Lerch J, Collins DL, Bernasconi A. Whole-brain voxel-based statistical analysis of gray matter and white matter in temporal lobe epilepsy. Neuroimage. 2004; 23:717–723.
Article6. Bonilha L, Rorden C, Halford JJ, Eckert M, Appenzeller S, Cendes F, et al. Asymmetrical extra-hippocampal grey matter loss related to hippocampal atrophy in patients with medial temporal lobe epilepsy. J Neurol Neurosurg Psychiatry. 2007; 78:286–294.
Article7. Keller SS, Wilke M, Wieshmann UC, Sluming VA, Roberts N. Comparison of standard and optimized voxel-based morphometry for analysis of brain changes associated with temporal lobe epilepsy. Neuroimage. 2004; 23:860–868.
Article8. Bonilha L, Edwards JC, Kinsman SL, Morgan PS, Fridriksson J, Rorden C, et al. Extrahippocampal gray matter loss and hippocampal deafferentation in patients with temporal lobe epilepsy. Epilepsia. 2010; 51:519–528.
Article9. Bonilha L, Rorden C, Castellano G, Pereira F, Rio PA, Cendes F, et al. Voxel-based morphometry reveals gray matter network atrophy in refractory medial temporal lobe epilepsy. Arch Neurol. 2004; 61:1379–1384.
Article10. Keller SS, Mackay CE, Barrick TR, Wieshmann UC, Howard MA, Roberts N. Voxel-based morphometric comparison of hippocampal and extrahippocampal abnormalities in patients with left and right hippocampal atrophy. Neuroimage. 2002; 16:23–31.
Article11. Pail M, Brázdil M, Marecek R, Mikl M. An optimized voxel-based morphometric study of gray matter changes in patients with left-sided and right-sided mesial temporal lobe epilepsy and hippocampal sclerosis (MTLE/HS). Epilepsia. 2010; 51:511–518.
Article12. Bonilha L, Rorden C, Castellano G, Cendes F, Li LM. Voxel-based morphometry of the thalamus in patients with refractory medial temporal lobe epilepsy. Neuroimage. 2005; 25:1016–1021.
Article13. Guye M, Régis J, Tamura M, Wendling F, McGonigal A, Chauvel P, et al. The role of corticothalamic coupling in human temporal lobe epilepsy. Brain. 2006; 129:1917–1928.
Article14. Keller SS, Cresswell P, Denby C, Wieshmann U, Eldridge P, Baker G, et al. Persistent seizures following left temporal lobe surgery are associated with posterior and bilateral structural and functional brain abnormalities. Epilepsy Res. 2007; 74:131–139.
Article15. Mueller SG, Laxer KD, Cashdollar N, Buckley S, Paul C, Weiner MW. Voxel-based optimized morphometry (VBM) of gray and white matter in temporal lobe epilepsy (TLE) with and without mesial temporal sclerosis. Epilepsia. 2006; 47:900–907.
Article16. Nelissen N, Van Paesschen W, Baete K, Van Laere K, Palmini A, Van Billoen H, et al. Correlations of interictal FDG-PET metabolism and ictal SPECT perfusion changes in human temporal lobe epilepsy with hippocampal sclerosis. Neuroimage. 2006; 32:684–695.
Article17. Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007; 38:95–113.
Article18. Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005; 26:839–851.
Article19. Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000; 11:805–821.
Article20. Jack CR Jr, Theodore WH, Cook M, McCarthy G. MRI-based hippocampal volumetrics: data acquisition, normal ranges, and optimal protocol. Magn Reson Imaging. 1995; 13:1057–1064.
Article21. Coan AC, Appenzeller S, Bonilha L, Li LM, Cendes F. Seizure frequency and lateralization affect progression of atrophy in temporal lobe epilepsy. Neurology. 2009; 73:834–842.
Article22. Riederer F, Lanzenberger R, Kaya M, Prayer D, Serles W, Baumgartner C. Network atrophy in temporal lobe epilepsy: a voxel-based morphometry study. Neurology. 2008; 71:419–425.
Article23. Li J, Zhang Z, Shang H. A meta-analysis of voxel-based morphometry studies on unilateral refractory temporal lobe epilepsy. Epilepsy Res. 2012; 98:97–103.
Article24. Brázdil M, Marecek R, Fojtíková D, Mikl M, Kuba R, Krupa P, et al. Correlation study of optimized voxel-based morphometry and (1)HMRS in patients with mesial temporal lobe epilepsy and hippocampal sclerosis. Hum Brain Mapp. 2009; 30:1226–1235.
Article25. Scanlon C, Mueller SG, Cheong I, Hartig M, Weiner MW, Laxer KD. Grey and white matter abnormalities in temporal lobe epilepsy with and without mesial temporal sclerosis. J Neurol. 2013; 260:2320–2329.
Article26. Hetherington HP, Kuzniecky RI, Vives K, Devinsky O, Pacia S, Luciano D, et al. A subcortical network of dysfunction in TLE measured by magnetic resonance spectroscopy. Neurology. 2007; 69:2256–2265.
Article27. Mueller SG, Laxer KD, Barakos J, Cheong I, Finlay D, Garcia P, et al. Involvement of the thalamocortical network in TLE with and without mesiotemporal sclerosis. Epilepsia. 2010; 51:1436–1445.
Article28. DeCarli C, Hatta J, Fazilat S, Fazilat S, Gaillard WD, Theodore WH. Extratemporal atrophy in patients with complex partial seizures of left temporal origin. Ann Neurol. 1998; 43:41–45.
Article29. Labate A, Cerasa A, Gambardella A, Aguglia U, Quattrone A. Hippocampal and thalamic atrophy in mild temporal lobe epilepsy: a VBM study. Neurology. 2008; 71:1094–1101.
Article30. Bonilha L, Rorden C, Appenzeller S, Coan AC, Cendes F, Li LM. Gray matter atrophy associated with duration of temporal lobe epilepsy. Neuroimage. 2006; 32:1070–1079.
Article31. Dabbs K, Becker T, Jones J, Rutecki P, Seidenberg M, Hermann B. Brain structure and aging in chronic temporal lobe epilepsy. Epilepsia. 2012; 53:1033–1043.
Article32. Faeth WH, Walker AE, Andy OJ. The propagation of cortical and subcortical epileptic discharge. Epilepsia. 1954; 3:37–48.
Article33. Santana MT, Jackowski AP, da Silva HH, Caboclo LO, Centeno RS, Bressan RA, et al. Auras and clinical features in temporal lobe epilepsy: a new approach on the basis of voxel-based morphometry. Epilepsy Res. 2010; 89:327–338.
Article34. Barbas H. Connections underlying the synthesis of cognition, memory, and emotion in primate prefrontal cortices. Brain Res Bull. 2000; 52:319–330.
Article35. Hermann B, Seidenberg M. Executive system dysfunction in temporal lobe epilepsy: effects of nociferous cortex versus hippocampal pathology. J Clin Exp Neuropsychol. 1995; 17:809–819.
Article36. Lieb JP, Dasheiff RM, Engel J Jr. Role of the frontal lobes in the propagation of mesial temporal lobe seizures. Epilepsia. 1991; 32:822–837.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prognosis of Temporal Lobectomy for Temporal Lobe Epilepsy Patients with Mental Retardation
- Ictal Vomiting Associated with Temporal Lobe Epilepsy of Dominant Hemisphere
- Clinical Correlations between Duration of Epilepsy and Anticonvulsant Treatment Response in Temporal Lobe Epilepsy
- Evaluation of Memory Impairment in Patients with Temporal Lobe Epilepsy Using the Wechsler Memory Scale
- The Clinical Analysis of Surgical Treatment in the Medically Intractable Seizure